BACKGROUND AND AIMS
Fetal macrosomia, a complication of gestational diabetes (GD), is associated with negative maternal and neonatal outcomes. Macrosomic newborns have increased risks of respiratory distress and neonatal hypoglycaemia, and are at a high risk of developing obesity and diabetes. Factors associated with macrosomia include maternal obesity/being overweight, high lipid and glucose concentrations, and older maternal age. Its prevalence is increasing worldwide simultaneously with the rates of obesity and diabetes, but its lifelong impact is not yet fully understood.1,2 Animal models of GD and macrosomia are necessary to study both conditions. Very few transgenic strains mimic GD and only diabetes (db)-/-strains show the poor fetal outcome of macrosomia.3 We have observed apparent macrosomia in the offspring of suppressor of cytokine signalling 2 (SOCS2) knockout mice (SOCS2-/-). SOCS2, through the janus kinase and signal transducers and activators of transcription (JAK/STAT) pathway, acts by mediating cytokine responses to control growth, development, metabolism, and immunity.4 The SOCS2-/- mouse, a model of gigantism, shows normal size at birth, and a progressive, proportional increase after weaning, without fat accumulation.5
Glucose impairment has also been described, although there are discrepancies among authors.6,7 To our knowledge, macrosomia and delivery problems have not been previously reported in the SOCS2-/- model before. Therefore, we aimed to evaluate the SOCS2-/- mouse as a potential model for fetal macrosomia.
MATERIAL AND METHODS
As part of routine colony management, pregnant SOCS2-/- mice were monitored. If an inability to give birth was detected, the mice were humanely euthanised. Mothers’ age and body weight (BW) were obtained. BW, head length, lateral height, dorsolateral diameter, abdomen width, and body length were measured in undelivered and delivered (control group) neonates. The mouse colony archive (May 2015–July 2017) was also reviewed. To compare groups, Mann-Whitney U tests and Student’s t-tests were assessed and bivariate correlations (Spearman) were performed. A two-tailed p<0.05 was considered significant.
RESULTS
A total of 21 pregnant females and their 126 (120 undelivered, 6 delivered) offspring were analysed. Evident dystocia was observed in all mothers (Figure 1A) and all litters seemed to be at an immature stage. Undelivered neonates were 40% heavier than delivered neonates (1.53 ± 0.18 g versus 1.08 ± 0.14 g; p<0.01) and had longer heads (1.41 [0.9–2.0] cm versus 1.17 [1.0–1.4] cm; p<0.024) (Figure 1B). No significant differences were obtained for abdomen width. Aberrant forms, like malformations and extremely big fetuses, were identified in 13% of the necropsied mothers. A total of 154 pregnancies were analysed from the mouse archive. Low rates of successful pregnancies (39%) and high rates of perinatal (19%) and maternal mortalities (56%) were identified. An age dependent role was observed for the rate of successful pregnancies (R: -0.47; p<0.001) and maternal mortality (R: 0.74; p<0.001).
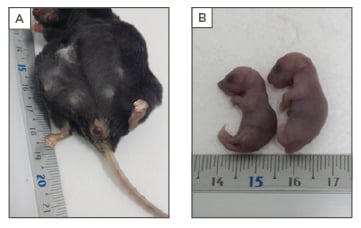
Figure 1: SOCS2-/- macrosomic phenotype.
A) A SOCS2-/- mother with delivery problems due to a severe fetal dystocia; B) Evident differences between the delivered and undelivered SOCS2-/- neonate littermates were noted.
SOCS2: suppressor of cytokine signalling 2.
CONCLUSION
Undelivered neonates were bigger than their delivered littermates and their size caused their mothers’ inability to give birth. Older females had higher maternal mortalities and lower birth success rates. We hypothesise that macrosomia and mothers’ maturity is associated with subsequent gestational diabetes. Further studies will be performed to evaluate the presence of diabetes and the role of SOCS2 in this macrosomia model.








