BACKGROUND
Diabetic foot ulcers (DFU) are usually chronic wounds that present in the inflammatory phase of the wound healing process.1 In the early inflammation phase, proinflammatory or classically activated (M1) macrophages secrete proinflammatory cytokines and ‘clean’ the ulcer by phagocytosing bacteria and debris. As the inflammation resides, macrophages undergo a transition to an anti-inflammatory and healing phenotype (M2 or alternatively activated macrophages).1,2,3 Diabetic animal wound studies have shown a delayed macrophage phenotype transition and an increased M1/M2 macrophage ratio.4,5,6The aim of the current study was to examine the macrophage phenotype in the skin of patients with diabetes with and without DFU, and to look for potential differences in skin macrophages in the forearm and foot of patients with DFU.
METHOD
A total of 20 patients with diabetes (10 with chronic noninfected DFU and 10 without DFU) and 12 healthy controls were recruited. Forearm 3 mm skin punch biopsies were obtained from all participants. In addition, punch biopsies from the borders of the foot ulcers were obtained from patients with DFU. Skin biopsies were fixed in formaldehyde, embedded in paraffin, and sections were cut for immunohistochemistry. Cluster of differentiation (CD)64 was used as a marker for the identification of M1 macrophages and CD163 as a marker of M2 macrophages. The ankle–brachial index was measured and values ≤0.90 were considered indicative of peripheral arterial disease, while diabetic peripheral neuropathy diagnosis was based on neuropathy symptom score and neuropathy disability score.
RESULTS
The three groups of participants did not differ in terms of age and sex. The two diabetic cohorts did not differ in terms of diabetes duration and glycaemic control. None of the participants had peripheral arterial disease, while all patients with DFU and nine patients (90%) with diabetes without DFU had diabetic peripheral neuropathy. The number of CD64+ and CD163+ cells from the forearm biopsies differed significantly between the three groups of participants (Table 1); subanalysis showed that patients with DFU had significantly higher numbers of CD64+ cells when compared with patients without DFU and healthy participants. Participants with DFU and without DFU had significantly higher numbers of CD163+cells when compared with healthy individuals in the forearm biopsies. The number of CD64+ and CD163+cells did not differ between the forearms and feet of patients with DFU: 5.8 (5.3, 6.4) versus 6.0 (5.5, 11.7), p=0.139; and 6.5 (5.2, 7.5) versus 7.0 (4.5, 8.3), p=1.000, respectively.
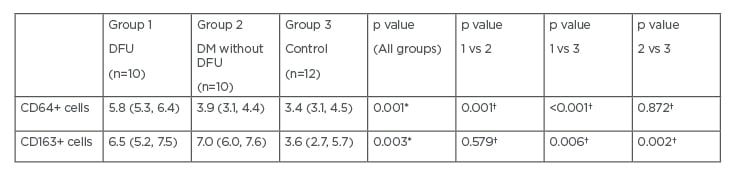
Table 1: CD64 and CD163 positive cells in forearm biopsies.
*p values for comparisons between all three groups by Kruskal–Wallis H test.
†p values for comparisons between pairs of groups (1 vs 2, 1 vs 3, and 2 vs 3) by Mann–Whitney U test.
CD: cluster of differentiation; DFU: diabetic foot ulcers; DM: diabetes mellitus; vs: versus.
CONCLUSION
There was increased inflammation in the skin of patients with DFU when compared with patients with diabetes without DFU, and with healthy individuals. In the forearms and feet of individuals with DFU, similar macrophage phenotype, which is associated with a chronic proinflammatory state, was observed. This notion could suggest that increased inflammation in the skin of patients with diabetes either results in foot ulceration, or impairs normal wound healing.Further, larger clinical studies are needed to clarify whether inflammatory cytokine inhibition or modification of macrophage phenotype could be a promising therapeutic approach for chronic refractory DFU.








