Speakers: Kilian Eyerich,¹,² Rik Lories³
1. Division of Dermatology and Venereology, Karolinska Institutet, Stockholm, Sweden
2. Technical University of Munich, Germany
3. Department of Development and Regeneration KU Leuven, Clinical Division of Rheumatology, UZ Leuven, Belgium
Disclosure: Eyerich has received honoraria from AbbVie, Almirall, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Janssen, Leo, Novartis, Sanofi, and UCB; and is a shareholder and co-founder of Dermagnostix. Rik Lories has received research funding from Excellence of Science financing FWO/FNRS, Innovative Medicines Initiative-2, KU Leuven, Flanders Research Foundation (FWO), Horizon 2020 – Marie-Curie ITN, and VIB (Flanders Institute for Biotechnology); and KU Leuven has received honoraria or research grants on behalf of Rik Lories from AbbVie, Amgen, Biosplice Therapeutics (Samumed), Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, Galapagos, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Sandoz, and UCB.
Acknowledgements: Medical writing assistance was provided by Fiona Powell of Excerpta Medica, Amsterdam, the Netherlands.
Support: The symposium and publication of this article were funded by Janssen. The views and opinions expressed are those of the speakers and not necessarily of Janssen.
Citation: EMJ Dermatol. 2021;9[Suppl 5]:2-8.
Meeting Summary
This Janssen-sponsored live satellite symposium, entitled ‘Where do we begin? The key role of the IL-23 pathway in psoriasis and psoriatic arthritis,’ took place at the 6th World Psoriasis and Psoriatic Arthritis Conference (WPPAC) 2021. The symposium focused on the role of the IL-23 pathway in the management of psoriasis and psoriatic arthritis (PsA). Kilian Eyerich and Rik Lories presented an overview of the IL-23 axis as a driver of pathophysiology in psoriasis and PsA, providing a comprehensive rationale for the therapeutic targeting of IL-23.
Eyerich evaluated the latest data to emerge following IL-23 inhibition in patients with psoriasis, noting the high rates of sustained Psoriasis Area Severity Index (PASI) responses, including the continued efficacy demonstrated in a subset of patients following treatment withdrawal. He discussed the modification of immune memory within the skin as a potential explanation for the activity observed following IL-23 inhibition in patients with psoriasis.
Lories highlighted the heterogeneous nature of PsA and the challenges conferred by the chronicity and progressive damage associated with the condition. He evaluated recent clinical data of IL-23 inhibition in patients with active PsA, with particular attention given to the progressively increasing rates of American College of Rheumatology (ACR) responses observed through 2 years.
With improvements in clinical parameters and patient-reported outcomes paralleled by a reassuring long-term safety profile, the faculty emphasised the need for optimised treatment positioning to facilitate a personalised management strategy for patients.
Where It All Started
Kilian Eyerich and Rik Lories
The IL-23 cytokine pathway is heavily involved in the pathogenesis of both psoriasis and PsA.1-4 Psoriasis is driven by an epidermal immune response initiated by the release of danger signals, such as antimicrobial peptides, and recognition of cellular damage. This triggers the release of cytokines including IL-23, which acts as a master regulator, driving the differentiation and proliferation of T-helper (Th) 17 cells, and their secretion of pro-inflammatory cytokines including IL-17A, IL-17F, IL-21, IL-22, and TNF-α. These cytokines are the hallmark of psoriasis pathology, promoting keratinocyte activation and proliferation, epidermal hyperplasia, and the recruitment of innate immune cells into the epidermis, with the subsequent release of antimicrobial peptides creating a self-sustaining pro-inflammatory cycle (Figure 1).1,2,5,6
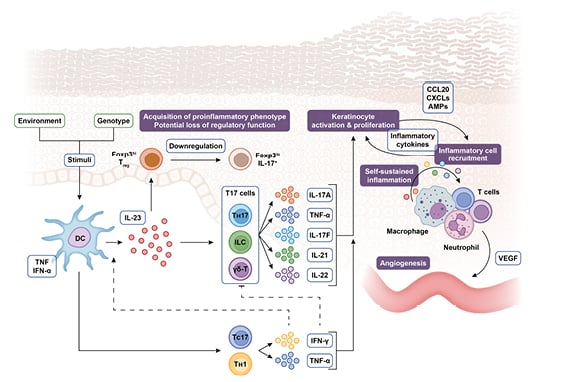
Figure 1: The steering role of IL-23 in the pathogenesis of psoriasis.
AMP: antimicrobial peptide; CCL: chemokine (C-C motif) ligand; CXCL: (C–X–C motif) ligand; DC: dendritic cell; Foxp3: forkhead box P3; IFN: interferon; ILC: innate lymphoid cell; TC: T cytotoxic cell; TH: T helper cell; TReg: regulatory T cell; VEGF: vascular endothelial growth factor; γδ-T: γδ T cell.
Adapted from Nestle et al.,⁵ Krueger and Bowcock,⁷ Bovenschen et al.,⁸ Leung et al.,⁹ Zhu et al.,10 Zúñiga et al.,11 Cai et al.,⁶ and Gaffen et al.¹
The role of IL-23 as a driver of effector cytokine secretion by Th17 cells is also key in steering the development of PsA,4 and multiple cell types are capable of cytokine release within the joint following IL-23 stimulation.12,13 Furthermore, the entheses contain unconventional T-cell populations that are thought to play a role in the monitoring of local tissues. These cells may react to localised microdamage, creating an amplifying pro-inflammatory loop which drives the pathological pathways characteristic of arthritis.14-16
With the role of IL-23 as a key steerer of pathogenesis well established, this cytokine presents an attractive cross-disciplinary therapeutic target; this has led to the development of several inhibitors of the p19 subunit of IL-23. Of these, guselkumab, tildrakizumab, and risankizumab are approved by the European Medicines Agency (EMA) for the treatment of moderate-to-severe plaque psoriasis, with guselkumab currently the only IL-23 inhibitor approved by the EMA for patients with active PsA who responded inadequately or were intolerant to a previous disease-modifying anti-rheumatic drug.17-19
The IL-23 Pathway: Navigating the Latest Updates in Skin Research
Kilian Eyerich
Recent data to emerge from clinical trials of IL-23 inhibitors for the treatment of psoriasis have highlighted some distinguishing features of this therapeutic approach.
IL-23 inhibition has been shown to induce tight and long-term disease control in the majority of patients. In the Phase III trial VOYAGE 1, patients with moderate-to-severe plaque psoriasis were randomised to guselkumab 100 mg at Weeks 0, 4, 12, and then every 8 weeks (q8w); placebo at Weeks 0, 4, and 12, followed by guselkumab 100 mg at Weeks 16, 20, and then q8w; or adalimumab 80 mg at Week 0, followed by 40 mg at Week 1 then 40 mg q2w through to Week 47. Of the patients who received guselkumab from Week 0, PASI 90 responses were achieved by 82% (as observed analysis) at Week 52 and 86% at Week 252 (Figure 2).20 Furthermore, treatment with guselkumab led to complete skin clearance, which was maintained for over 3 years in 23% of patients within VOYAGE 1.21 Similarly, in the pooled Phase III trials reSURFACE 1 and 2, high rates of PASI 75 (100%), 90 (71–73%), and 100 (29–37%) responses were reported at Week 28 for patients with moderate-to-severe psoriasis who received tildrakizumab 100 mg or 200 mg at Weeks 0 and 4, and then q12w. These response rates were also sustained through Week 244,22 suggesting long-term control is a class effect of IL-23 inhibition.
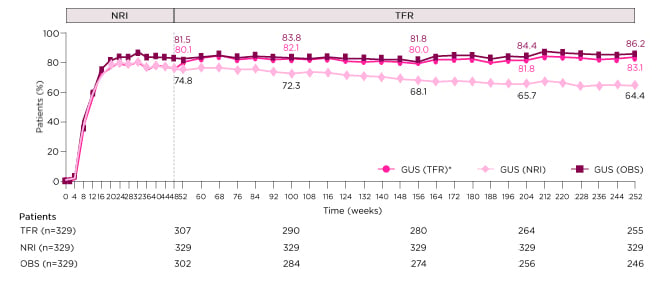
Figure 2: PASI 90 response through Week 252 in patients randomised to guselkumab from baseline in the Phase III clinical trial VOYAGE 1.
*NRI through Week 48, then TFR beyond Week 48.
GUS: guselkumab; NRI: non-responder imputation; OBS: as observed; PASI 90: ≥90% improvement in Psoriasis Area and Severity Index response; TFR: treatment failure rules.
Adapted from Griffiths et al.20
Secondly, the effects of IL-23 have been shown to extend beyond the withdrawal of treatment. This is clearly illustrated by data from VOYAGE 2, a Phase III trial in which patients who achieved ≥PASI 90 response at Week 28 having received guselkumab (100 mg at Weeks 0, 4, and then q8w) were re-randomised to guselkumab or the placebo. Although a higher proportion of patients who continued to receive guselkumab maintained PASI 90 response through Week 48 than those who had treatment withdrawn (89% versus 37%), more than one-half of the re-randomised placebo group maintained PASI 75 response, with 20% maintaining PASI 100 through Week 48.23 This effect was still noticeable at Week 72 when 12% of patients who had treatment withdrawn maintained a PASI 90 response. The maintenance of PASI response following treatment withdrawal was associated with the suppression of IL-17A, IL-17F, and IL-22, supporting the role of IL-23 in driving Th17 and Th22 responses.24 Additionally, shorter disease duration, lower BMI, and lower levels of IL-17F and macrophage inflammatory protein 1β at baseline, together with PASI 100, Investigator’s Global Assessment (IGA) 0, and higher guselkumab concentration at Week 28, were predictive of maintaining PASI 90 response following the withdrawal of guselkumab.25 A similar trend was reported in the IMMhance Phase III trial, where patients with moderate-to-severe plaque psoriasis who achieved a static Physician’s Global Assessment (sPGA) 0/1 response to risankizumab (150 mg at Weeks 0, 4, and 16) at Week 28 were re-randomised to risankizumab (q12w) or the placebo. Of the patients who had IL-23 inhibition withdrawn, 61% maintained sPGA at Week 52 versus 87% of patients who continued risankizumab. This dropped to 7% and 81% respectively at Week 104. Furthermore, PASI 90 responses were maintained through Week 52 by 86% of patients who continued risankizumab versus 52% of patients who had treatment withdrawn. By Week 104, PASI 90 response rates were 78% and 4%, respectively.26
The concept of localised immune memory is now well established, with non-circulating tissue-resident memory (TRM) T cells identified in epithelial barrier tissues including the skin. Atypical activation of these persistent TRM T cells has been shown to drive inflammatory diseases, such as psoriasis, through the secretion of IL-17.
Furthermore, these memory cells have been identified in non-lesional skin, suggesting they are likely to be associated with disease relapse.27,28 Indeed, the presence of these distinct, specialised effector memory cells is evidenced in psoriasis by the continued recurrence of plaques at the same sites. It is feasible, therefore, that long-term control of psoriasis may depend on the suppression of these TRM T cells.27 Potential modification of immune memory within the skin by IL-23 inhibitors may explain the long-term causative effects observed in patients with psoriasis. The mechanisms behind the tight and long-term clinical responses generated by IL-23 inhibition are now being investigated in the GUIDE trial, which is evaluating differences between ‘super-responders’ and non-responders in an effort to predict response to IL-23 inhibition and evaluate future therapeutic strategies.29
Learnings from IL-23 Pathway Data in Psoriatic Arthritis
Rik Lories
IL-23 is also established as a key driver of pathology in PsA,3,4 although this is a more heterogeneous disease than psoriasis, with the added dimension of progressive joint damage and loss of function impacting management strategies. Interestingly, despite common pathological pathways, there is only a modest correlation between the severity of skin and joint disease.30,31
PsA is characterised by diverse musculoskeletal and skin manifestations. The majority of patients present with peripheral arthritis and/or psoriasis, with dactylitis and enthesitis reported in approximately one-third to one-half of patients,32–36 and this is reflected in the selection criteria of PsA clinical trials.
IL-23 inhibition has demonstrated efficacy in patients with active PsA in Phase II clinical trials, with significantly and consistently higher rates of ACR20 responses reported at Week 24 versus the placebo in trials of guselkumab (100 mg at Weeks 0, 4, and then q8w; 58% ACR20), risankizumab (150 mg at varying frequency; 43–59% ACR20), and tildrakizumab (20–200 mg every 4 weeks [q4w] or q12w; 71–80% ACR20).37-40
The more stringent ACR50 response is often considered more clinically relevant by rheumatologists; in Phase III clinical trials, this was achieved by 36% of biologic-naïve or biologic-experienced patients with active PsA at Week 24 following guselkumab 100 mg q4w, versus 9% of patients who received the placebo in the Phase III DISCOVER-1 trial. Interestingly, the response rate rose to 54% in the guselkumab group at Week 52, and this trend for progressive improvement was echoed in the ACR70 response rate, which rose from 20% at Week 24 to 29% at Week 52.41 These data are supported by longer-term follow-up within the Phase III DISCOVER-2 trial, in which biologic-naïve patients with active PsA received guselkumab (100 mg q8w or q4w) or the placebo with cross-over to guselkumab 100 mg q4w at Week 24. ACR50 responses at Week 24 were reported for 32% and 33% of patients who received guselkumab q8w and q4w, respectively, and 14% of patients who received the placebo. However, at Week 100, response rates had risen to 55%, 56%, and 48%, respectively, with similar trends noted for ACR70 responses, suggesting responses to IL-23 inhibition have the potential to grow over time (Figure 3).42
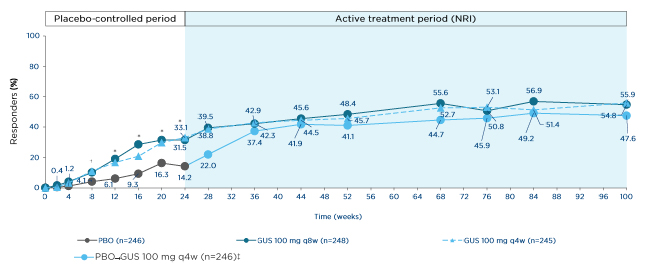
Figure 3: ACR50 response through Week 100 in biologic-naïve patients with psoriatic arthritis in the Phase III clinical trial DISCOVER-2.
*p≤0.001
†p<0.05.
‡Includes randomised patients who received ≥1 dose of study drug.
GUS: guselkumab; NRI: non-responder imputation; PBO: placebo; q4w: every 4 weeks; q8w: every 8 weeks.
Adapted from McInnes et al.42
The Phase IIIB COSMOS trial evaluated guselkumab (100 mg Weeks 0, 4, and then q8w) in a more challenging-to-treat population of patients with active PsA and inadequate response to TNF inhibitor. At Week 24, ACR20 responses were observed in 48% of patients who received guselkumab versus 20% of those who received the placebo.43
Long-term efficacy data clearly demonstrate the clinical benefits of extended IL-23 inhibition, and it is reassuring to note that the safety profile of continuous guselkumab remained manageable and stable through 5 years in the pooled VOYAGE 1 and 2 trials, with low rates of adverse events.44 These data facilitate the long-term management of chronic conditions by IL-23 inhibition.
Managing the complexities of pain and fatigue are two additional challenges associated with the treatment of rheumatological diseases and this is beginning to be reflected in the endpoints of clinical trials. Within the DISCOVER 1 and 2 trials, IL-23 inhibition demonstrated independent treatment effects on fatigue at Week 24, with 54–61% of patients who received guselkumab 100 mg q8w achieving a clinically meaningful improvement (≥4 points) in FACIT-Fatigue from baseline, versus 35–46% of patients who received the placebo.45
Summary
Disease modification is a well-established concept in rheumatology, with the aim of preventing long-term structural damage. The high rates of sustained clinical response that are observed with IL-23 inhibition in patients with psoriasis suggest that this may also be a possibility in dermatology. However, although the emerging data are impressive, whether the presence of localised immune memory cells will permit symptomatic and chronic disease modification in the skin remains to be determined.
While IL-23 inhibition has demonstrated encouraging clinical activity in patients with PsA, the level of benefit may differ depending on the extent of joint damage in patients with advanced disease. Intriguingly, however, there is evidence to suggest that the joint may be partially restored if inflammation is sufficiently suppressed, and it will be interesting to establish whether IL-23 inhibition could play a role in this process.
As clinical data establish the importance of IL-23 inhibition in managing psoriasis and PsA, it will be vital to define the role and positioning of these therapies to optimise the personalisation of therapeutic strategies.







