Meeting Summary
Psoriasis development involves genetic and environmental factors triggering inflammatory cascades involving keratinocytes, neutrophils, dendritic cells, and T-helper (Th) cells. Cytokines and other cell messengers involved in psoriasis pathogenesis can be targeted by therapy. This symposium focussed on the mechanism of action (MoA) and efficacy of fumaric acid esters (FAE) and IL-23 inhibitors. The FAE dimethyl fumarate (DMF) works at a number of levels, including blockage of signal transduction factors Nrf2, Nf-κB, STAT1/STAT3, and HIF-1α; mediation of stress-related glutathione stimulating hormone (GSH); and reduction of neutrophil recruitment, adhesion, and migration. FAE formulations can be DMF alone or a mixture of several types; however, because the former has proved as effective as the latter, DMF may be the only main active ingredient. Long-term studies have proven FAE to be efficacious and safe for many patients with moderate-to-severe plaque psoriasis. FAE can be combined with other psoriasis-targeting therapies and coprescribed with medications for comorbid conditions as there is no significant clinical evidence of drug–drug interactions. An alternative therapy is cytokine-targeting monoclonal antibodies such as those that inhibit IL-23. These work at an early stage of the psoriasis damage cascade, and while they take a number of weeks to show efficacy, the efficacy of treatment is maintained for a long time. This is important as time free of symptoms is one the most desired factors by both patients and prescribers. The symposium concluded with an interactive session in which the audience discussed treatment regimens and safety, and the panel discussed potential future use and studies of FAE and IL-23 inhibitors.
Dimethyl Fumarate, a Small Molecule for the Treatment of Moderate-to-Severe Psoriasis
Professor Antonio Costanzo
Psoriasis Pathogenesis and Genetic Pre-Disposition
Development of psoriasis occurs due to interactions with epidermal keratinocytes and immune-system cells including T-lymphocytes, antigen-presenting dendritic cells, and neutrophils. Currently, variations in around 63 genes are implicated in increasing the chances of developing psoriasis, many of which involve immune system cell operation.1,2 These genes cover a range of functions including general response to stimuli; regulation and differentiation of lymphocytes; the Type 1 IFN pattern/recognition pathway; and regulation of the adaptive immune response and the IkBα/NF-κB cascade.2
In those with a genetic predisposition, psoriasis develops through a pathway of interactions following an environmental inflammatory trigger toward a keratinocyte, such as stress, trauma, or micro-organism action. This leads to the release of antimicrobial peptides, such as LL37, by stressed cells, which in turn prompts the release of DNA from both the stressed cell and the skin microbiome bacteria. LL37 and released DNA can form a complex, which can activate plasmacytoid dendritic cells via internal Toll-like receptors (TLR), which then instruct T-lymphocytes to become Th1 and Th17 cells and induce production of several cytokines.3,4 Further cytokine production can occur as the LL37/DNA complex can act as an auto-antigen by fitting into the groove of human leukocyte antigen-Cw6 that is then presented to the T-lymphocyte.4
Dimethyl Fumarate Mechanism of Action in Psoriasis
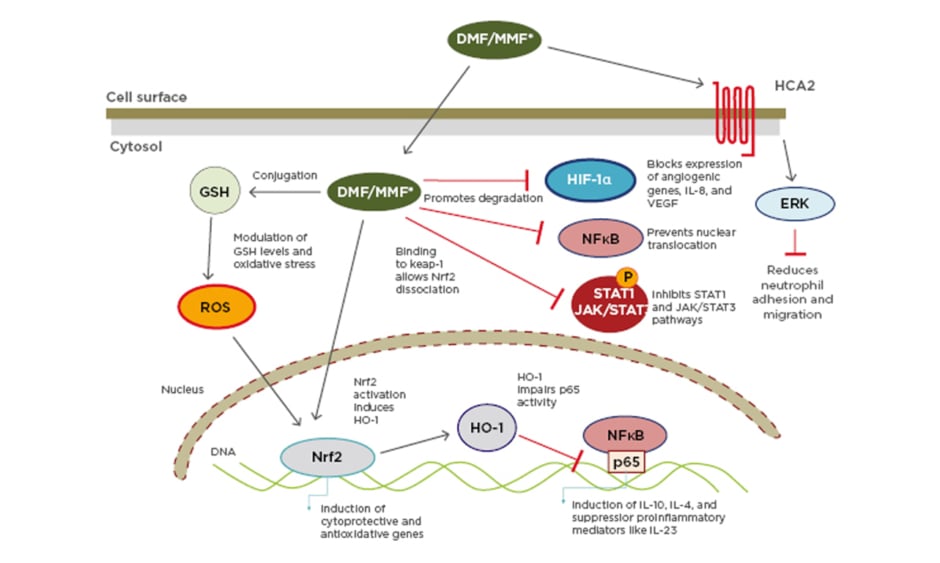
This cascade is important in the MoA of DMF, used alone or as a combination of FAE to treat psoriasis. FAE mediate their anti-psoriatic effects via activation of several cellular pathways that induce T-cell apoptosis or prevent release of proinflammatory cytokines (Figure 1).5
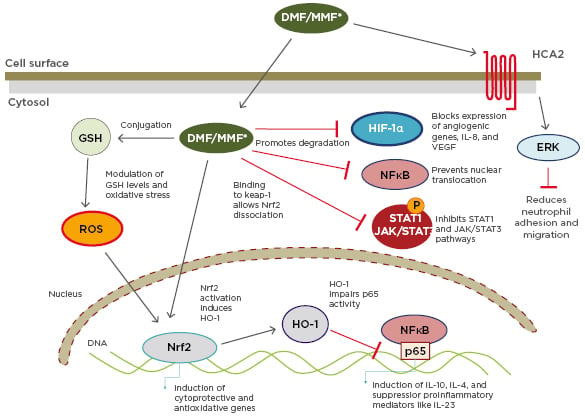
Figure 1: Multiple actions of dimethyl fumarate/monomethylfumarate.
DMF: dimethyl fumarate; ERK: extracellular signal-regulated kinase; GSH: glutathione stimulating hormone; HCA2: hydroxy-carboxylic acid receptor-2; HIF-1α: hypoxia-inducible factor 1-alpha; HO-1: haem oxygenase 1; JAK: janus kinase; Keap-1: Kelch-like ECH-associated protein-1; MMF: monomethylfumarate; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2: nuclear factor (erythroid-derived 2)-like 2; ROS: reactive oxygen species; STAT: signal transducers and activators of transcription.
Reproduced with permission from Brück J et al.5 A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Experimental Dermatology. 2018;27:611–624.
© 2018 The Authors. Experimental Dermatology Published by John Wiley & Sons Ltd.
DMF is taken orally and, following ingestion, is converted by gut esterases to monomethylfumarate (MMF), which is hydrolysed to fumaric acid and metabolised via the citric acid cycle. Methanol produced from DMF/MMF is oxidised to form CO2 upon exhalation, the main excretion route for FAE.5,6 DMF/MMF has no clinically significant drug–drug interactions with cytochrome p450 (CYP) enzymes or the P-glycoprotein drug transporter.7 This is crucial as psoriasis is associated with an increased risk of cardiovascular events8 and a patient may be taking medications that are metabolised by CYP/P-glycoprotein mechanisms.
Once DMF/MMF enters a cell, there are several different MoA. The main MoA is activation of the Nrf2 transcriptional pathway.9
Nrf2 is usually locked in the cytoplasm, but activation by DMF/MMF allows it to translocate to the nucleus where it activates a pathway of genes involved in antioxidant responses, detoxification, and the activity of cytoprotective anti-inflammatory factors. These factors include haem oxygenase-1, which has important anti-inflammatory properties toward cytokines including upregulating IL-10, and downregulating IL-6 and TNFα through other means, such as NF-κB signalling.9,10
Nf-κB can itself activate cytokine transcription; another MoA of DMF/MMF is the direct inhibition of NF-κB signalling. DMF/MMF actions may also be indirect, including blocking of cytokine-activated I-κB, a cytoplasmic complex that can induce degradation of a NF-κB inhibitor, releasing inflammatory transcription factors to the nucleus to induce inflammation. These pathways are important in activating the Th17 cells involved in psoriasis development.10,11
Extra or intra-cellular signals, for instance from DNA, can activate the intracellular complex known as the inflammasome. This complex can activate inflammatory cytokines and is critical in allowing antigen presentation. Blocking of the inflammasome by DMF/MMF means an antigen cannot come in with an adjuvant to be presented to the major histocompatibility complex.12,13 It is thus hypothesised that, in the presence of DMF, the ability of dendritic cells to properly present auto-antigens, including the aforementioned LL37, to T-lymphocytes is blocked.
Cell signalling can be disrupted by oxidative stress. Another MoA of DMF/MMF is reduction of GSH levels, which can lessen oxidative stress effects.10,14 A further action of DMF is to modulate transcription factors that are sensitive to oxidative stress such as hypoxia-inducible-1a and STAT3/STAT1.10 The STAT3/STAT1 cascade can be increased in keratinocytes through regulation of factors that sense both oxidative stress and reduced levels of oxygen.5,10 By inhibiting expression of genes regulated by these transcription factors, DMF can thus regulate inflammatory responses.10
Neutrophils, the first immune system cells found in the epidermis in psoriasis, are an additional source of complexes that can activate the vicious circle in disease development and persistence. They are activated by TLR and ‘danger’ signals, and can extrude DNA and antimicrobial peptides, which can lead to dendritic cell activation in psoriasis. MMF can modulate neutrophil adhesion, recruitment, and migration through blockage of hydroxycarboxylic acid receptor 2, a receptor important for neutrophil extravasation.15,16 As such, blocking neutrophils blocks development of psoriasis at a very early stage.
Summary
In conclusion, DMF/MMF can interject specific points in the pathogenesis of psoriasis involving several types of immune system cells along with the affected keratinocytes. While their full MoA remains to be elucidated, it is known that they can activate Nrf2; modulate GSH; block Nf-κB, HIF-1a, and STAT pathways; reduce neutrophil adhesion and migration; and modulate the inflammasome to block correct antigen presentation by dendritic cells.
Fumarates as First Systemic Treatment: Their Long-Term Efficacy
Professor Diamant Thąci
History and Current Use
In 1959, the chemist Dr Walter Schweckendiek developed fumarates as a treatment for psoriasis.17 Up until the last decade, FAE, as a mixture of several fumarates, was the first choice for systemic treatment of psoriasis in Germany.18 While there are now a number of new treatments, the German PsoBest registry still shows that approximately half of those who receive non-biological therapy receive a FAE, with currently many being switched to DMF alone.19
Fumaric Acid Esters Use
Patients who respond best to FAE include those with moderate-to-severe plaque psoriasis, but not those with psoriatic arthritis or nail psoriasis. FAE formulations can be a mixture of ingredients; however, there are different studies indicating that the main active ingredient appears to be DMF. For instance, in the BRIDGE trial comparing DMF alone to a compound containing DMF plus other FAE, while both were significantly more effective than a placebo, there was no significant difference between them, indicating that DMF is the active ingredient in terms of efficacy.20 In another study, the data showed that the efficacy remained unchanged in the majority of patients. Tolerability (e.g., gastrointestinal complaints and flushing) of the DMF drug was rated equal or better in most patients, showing that patients can switch form the traditional FAE mixture to the same dose of DMF with similar clinical relief but without any washout period.21
Fumaric Acid Ester Efficacy and Combinations With Other Therapies
Studies of FAE have shown them to be increasingly effective when used long-term. For instance, in one study, by 3 months, 31% of the patients were rated as ‘markedly improved and clear’, with 50% ‘slightly improved’. By 1 year, this was 76% and 14%, respectively. Percentage improvements continued past 3 years in those who remained on treatment.18 Continued efficacy was confirmed in a similar study in which Psoriasis Area and Severity Index (PASI) score improvements of at least 50%, 75%, and 90% was shown to be achieved by increasing percentages of participants over a period of years.22
Examination of patient records shows that many persist on long-term FAE treatment. A systematic review found FAE to have the longest persistence of treatment for non-biologic systemic therapies for psoriasis, showing discontinuation at a mean of 50.0 months, compared to methotrexate treatment discontinuation at 22.3 months.23
Patients with small patches of plaques may be hard to treat with systemic therapy alone but can be treated successfully when FAE is combined with another therapy such as phototherapy, topical, or other systemic drugs. As FAE has a relatively slow induction treatment therapy, combination therapy can also speed up the response. For instance, adding topical calcipotriol to FAE shortened time to achieve PASI 50 from 9 weeks to 3–4 weeks in one study. Additionally, the combination group needed lower FAE doses.24 Unlike many systemic therapies, FAE may be combined with phototherapy, and for those with psoriatic arthritis, with methotrexate.13
Fumarates and Co-medication
There is no evidence for FAE interaction with cytochrome P450 and the most common efflux and uptake transporters,7 and therefore no interactions are expected with medicinal products metabolised or transported by these systems. For example, no interaction between DMF and oral contraceptives has been found.25 As such, they are the drug of choice for people with comorbidities that require, for example, lipid modifying agents,26 as they do not interfere with metabolism of these drugs.
Interestingly, in two small studies, those with metabolic comorbidities treated with a FAE showed improvement in metabolic syndrome-associated biomarkers27 and in endothelial function.28 An in vitro study using rat heart endothelial cells found that DMF inhibited NF-κB and, in an in vivo rat model, reduced myocardial infarction size.29 Another study posited that DMF could potentially attenuate recurrence of stenosis after acute vascular injury.30 Further work is being carried out to investigate these findings.
Safety of Fumaric Acid Esters
During treatment with DMF, it is very important to conduct complete blood counts with differential before initiating treatment and every 3 months thereafter together with the quarterly visits. This is to assess tolerability in general and the level of leukocytes and lymphocytes in particular. Dosage modifications or discontinuation may be necessary if abnormalities in laboratory parameters are observed. If lymphocyte counts are between 700 and 1,000 cells/mL, treatment should be monitored and should be suspended if there are two consecutive readings of <700 cells/mL. Following discovery of a sparse number of occurrences of progressive multifocal leukoencephalopathy (PML) in those taking a FAE, median duration of FAE therapy to PML symptom onset or appearance of first PML brain lesion was 31 months (range 6–110), with median duration of lymphocytopenia to PML symptoms being 23 months (range 6–72).31 The median lymphocyte count at PML diagnosis was 414 cells/mL, and the authors concluded that if a person had lymphocytopenia over a long time, this might be a risk factor for PML.31
Following vaccination in people with multiple sclerosis taking DMF, one study found no decrease in the ability to mount an immune response.32
There are low rates of serious and severe infections and of malignancies in those receiving FAE, similar to or lower than with methotrexate, cyclosporine, or biologics.33 An in vivo study showed that DMF restored apoptosis sensitivity and inhibited tumour growth and metastasis in cutaneous T-cell lymphoma by targeting NF-κB.34 As such, it could be that FAE has a protective effect against malignancies, though this has yet to be tested.
Treatment Recommendations with Fumaric Acid Esters
As a summary of treatment recommendations, it is advised to start with a low dose of a FAE then increase to the optimal dose by monitoring efficacy and side-effects. A person can remain on a FAE for the long term as there are no indications of an increased risk of severe infection or malignancies. Switching between a FAE mixture and a DMF-only formulation does not seem to present any problems. Patients can receive vaccinations while being treated with DMF and most surgical interventions do not necessitate FAE discontinuation, other than those on the gastrointestinal system (as DMF is metabolised by gut esterases).
Summary
In conclusion, fumarates are an established, effective, and safe treatment option for people with moderate-to-severe psoriasis. They can be used in the short or long term and rarely interact with other medications. In those taking a FAE, long-term lymphocyte counts should be monitored.
Biological Treatment: Role of IL-23 Inhibition
Professor Stefano Piaserico
Development of Psoriasis Treatments
While historically psoriasis treatment predominantly consisted of sun exposure, treatment has advanced over the last 100 years with addition of FAE, methotrexate, retinoids, topical vitamin D, ciclosporin, and biologics.35
As discussed earlier, trauma, coupled with an associated genotype, can damage keratinocytes, leading to the expression of IFNγ and TNFα, activation of dendritic cells, and production of IL-23. IL-23 affects the differentiation of Th17 cells, which may produce IL-17, IL-22, TNFα, and IFNγ effector cytokines. IL-17 is also involved in recruiting neutrophils. The main consequence of these actions is keratinocyte activation and proliferation, which results in development of a psoriatic plaque.3,36,37 On the other hand, keratinocytes may also produce IL-23 via the actin polymerising molecule N-WASP demethylating the histone H3K9 that controls IL-23 expression. Stimuli for this include TNFα, DNA damage, and TLR activation, all components of the psoriasis-development pathway, as discussed above.38 This can induce further recruitment of Th17 cells and neutrophils, producing a pathological vicious cycle.
Treatment of Psoriasis
In the 1980s, psoriasis was viewed as a keratinocyte-driven disease and the treatment employed was to inhibit proliferation of epithelial tissue using methotrexate, phototherapy, and retinoids. It was then accidently discovered, in a patient using cyclosporine, that psoriasis had a strong immunological component. In the 1990s, psoriasis was positioned as an IL-12/Th1-mediated disease, and therefore therapy including TNFα blockers was used; however, this was not shown to be very effective. More recently, focus has been on psoriasis as an IL-23/Th17-mediated disease, with therapies including anti-IL-12/23 subunit p40, anti-IL-17, and anti-IL-23p19 monoclonal antibodies.
One such biologic used for psoriasis treatment is the IL-12/IL-23 inhibitor ustekinumab; however, blocking IL-12 may not be necessary. Work in human biopsy models has shown that IL-12p35 was not expressed within psoriatic skin lesions, whereas p40 in IL-12 and IL-23, along with IL-23p19, were significantly expressed.39 The IL-12 group is a complex family of cytokines. Blocking of IL-12p40 results in inhibition of several types of cytokines, including those involved in controlling inflammation.40 In a mouse model, IL-12 was shown to be protective against thicker plaques, therefore inhibition may be counterproductive.41 This means that exclusively blocking IL-23p19 results in more direct and stronger inhibition of inflammation with no diverse effects on Th1/Th17 regulation.41
Targeting IL-23
Activated dendritic cells can induce proliferation of Th1 and Th2 cells. However, the most important pathway in psoriasis pathogenies is activation of Th17 cells in the presence of IL-6 and TGFβ. In the absence of IL-23, these Th17 cells mature to a non-pathogenic type that produce IL-10 and IL-17, cytokines involved in inflammatory response regulation. In the presence of IL-23, Th17 cells mature to a pathogenic type that produce IL-17, IL-22, and IFNγ.42,43
With this in mind, it can be seen that IL-23 is an upstream molecule, regulating Th17 cells that will eventually produce IL-17, which will connect to the IL-17 receptor. In blocking the IL-17 receptor itself, or cytokines that appear as a result of IL-17 effects, such as TNFα, one can only target the end of the pathway. While the therapeutic effect is quick, frequent dosing is needed and there is potential for a quick relapse and rebound of psoriasis (Table 1). Blocking a mid-stream target like IL-17 itself also results in a quick onset of action and has the advantage of less frequent dosing; however, time in remission is not very long. Blocking upstream, for example by targeting IL-23, takes longer before an onset of action; however, dosing is less frequent, rebound is unlikely, and there is a longer remission time. For instance, long-term studies with the IL-23-targeting therapy tildrakizumab showed an excellent response over time and long-term maintenance of response. As the drug blocks IL-23 upstream, in a clinical trial PASI 75 was maintained in 42% of participants after 1 year from the last dose of treatment.50
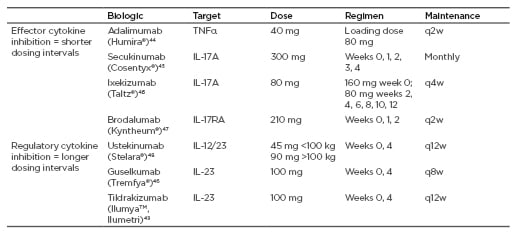
Table 1: Targets, dose, and regimen for biologic therapy.
q2(4/8/12)w: every 2 (4/8/12) weeks.
It is hypothesised that IL-23 inhibitors also have the potential to modify the course of the disease itself. This is because even when a psoriatic plaque is resolved, IL-17 and IL-22-producing cells remain in the epidermis and can be triggered into recruiting circulating T cells, producing a plaque relapse.51 As IL-23 inhibitors target upstream of these cells, IL-17 and IL-22 are potentially not produced and so do not remain following plaque remission, thus lessening the chances of a relapse.
Patient and Prescriber Choice
Choice of treatment needs to be directed by both the patient and the prescriber as their needs may be slightly different. A recent study found that the highest driver of a physician’s choice of treatment was reduction in percentage of body surface area affected, overall perception of fficacy, and maintenance of response. For a patient in this study, maintenance of response, along with reductions in lesion-associated symptoms and in redness, thickness, and scale, scored the highest when rating importance of a drug.52 Another study found that people with psoriasis ranked time free of symptoms and time to improvement over mode of frequency of administration, treatment cost, and ‘unintended life expectancy reduction resulting from treatment’.53
The Potential Positive Impacts of Inhibiting IL-17/IL-23/Th17 Cells Besides Psoriasis
The therapeutic value of IL-23/Th17 inhibitors may go beyond their use in psoriasis. Depression is an inflammatory disorder and it is known that IL-23 and IL-17 are important in inducing central nervous system inflammation.54 It is hypothesised that systemic effects of IL-23 inhibitors may lead to a reduction of inflammation in the central nervous system, potentially reducing the occurrence of depression.
Adipose tissue can worsen inflammation due to high IL-23 expression and recruitment of Th17 cells. Inhibition of the IL-23 pathway may therefore have positive consequences on metabolic dysfunction.55 Nonalcoholic fatty liver disease can be driven to nonalcoholic steatohepatitis by IL-17, which can eventually lead to sclerosis and liver cancer.56 Trials are currently being performed to see if inhibiting IL-17 in sclerotic patients can affect their liver status.
Use of an IL-23-specific inhibitor may be important in those with inflammatory bowel disease. Bowel mucosa, and integrity of the intestinal epithelial barrier, is protected by IL-17,57 meaning its removal will eventually disrupt this barrier leading to further penetration of bacteria and worsening of inflammation. Hence, in theory, using IL-17 blockers may not be good for those with inflammatory bowel disease.
Finally, it is hypothesised that blocking IL-23 has an impact on new bone formation. Blocking IL-23 results in a downstream block of IL-22 and pathogenic Th17 cells involved in bone loss pathogenesis.58 Blocking IL-23 may thus eventually reduce bone loss and osteoproliferation, a typical feature of psoriatic arthritis.58
Summary
Innate and adaptive immune cells work together to drive inflammation and induce psoriatic plaques. Pathogenic models of psoriasis emphasise the pivotal role of the IL-23/Th17 axis and blocking this axis might determine positive effects on psoriasis comorbidities. Use of IL-23-targeting therapies is highly effective, with sustained disease control over time. Pharmacodynamic effects of anti-IL-23p19 inhibition are longer than pharmacokinetic effects, thus the impact on psoriasis can continue many months after the drug is excreted. Additionally, there is the potential to modify the underlying disease with IL-23 inhibitors.
Interactive Question and Answer Session
The panel discussed how, with a FAE, most patients are started on three tablets, and the dose may be increased to up to six tablets if needed; in the long-term however, most are on one to two tablets assuming they respond well and tolerate the medication. Response and dosing do not seem to be weight related. Regarding side effects, FAE can cause dose-related gastrointestinal complaints, but these usually pass. It was remarked that while FAE have not been shown to be teratogenic according to the PsoBest registry, as no studies have been carried out in pregnant women, they cannot currently be recommended for such. No risk has been shown in postpartum women.
With biologics, TNFα inhibitors are still used regularly, especially for those with psoriatic arthritis. An IL-17 inhibitor is used when a quick response is needed, with all other patients receiving an IL-23 inhibitor. The panel also discussed that treatment of dermatitis with IL-17 and IL-23 inhibitors, and how technically these treatments may be beneficial for those with lichen planus. Finally, the panel posited that as IL-23 inhibitors may be able to limit establishment of IL-17/IL-22 memory cells responsible for relapses in psoriasis, trials are needed focussing on early stage treatment.
Chair’s Summation
Prof Thąci concluded by discussing the future research needed regarding the broad effects of fumarates in psoriasis and how both this class of drugs and IL-23 inhibitors should be considered for use in long-term therapy.