Meeting Summary
Prof Bieber opened the symposium by explaining that there has been a revolution in the management of patients with moderate-to-severe atopic dermatitis (AD) since the approval of the first biologic, dupilumab, in 2017. He explained that the symposium was part of an ongoing education programme ADstarted this year by Sanofi Genzyme and Regeneron. The goal of this programme is to support dermatologists to improve the management of patients with moderate-to-severe AD and bring together clinicians from different specialities to optimise the treatment of a range of diseases commonly occurring in patients with AD. Prof Guttman-Yassky reviewed the latest developments in the understanding of the pathophysiology of AD, particularly the recognition of its systemic nature and the central role of type 2 cytokine activation, and how this has led to the development of novel treatments. Prof Bieber explained the need to evaluate AD patients with objective clinical assessments together with subjective patient-reported outcomes (PRO) to better understand the impact of AD on the patient and their quality of life, and how to plan treatment to improve both aspects. The management challenge posed by the persistent nature of AD, which can last for many years in some patients, was addressed by Prof Thaçi. He highlighted the need for effective, safe, and well-tolerated long-term systemic treatment due to the chronic nature of AD and the limited use of immunosuppressive agents because of their benefit–risk profile. He also reported the long-term efficacy and safety data for dupilumab.
From Pathophysiology to Disease Burden in Atopic Dermatitis
Professor Emma Guttman-Yassky
AD is the most common inflammatory skin disease, affecting 3–7% of adults and 15–25% of children.1-5 Between 20% and 30% of patients have moderate-to-severe disease5,6 and there is a large unmet need for more effective and safe treatments for long-term disease control.7
AD has a complex multifactorial pathogenesis, with abnormalities in adaptive immune responses, particularly in the Th2 and Th22 axes, and also barrier abnormalities, with contributions to each from the microbiome.1,8-10 Until recently, there were two competing pathogenic hypotheses. The epidermal-based model proposed that AD is a disease of fixed (genetic) epidermal barrier defects that may trigger abnormal keratinocyte hyperplasia and secondary immune activation.1,11-13 Alternatively, the immune-based model proposed that the abnormal epidermal phenotype in lesional AD skin is initiated by increased expression of cytokines that induce epidermal abnormalities.1,9,14
AD lesions are always accompanied by immune activation, particularly type 2 cytokine activation, such as IL-4, IL-13, and IL-31.15 In acute lesions, there is a very large increase in IL-4, IL-13, and IL-31 compared with non-lesional skin, in addition to multiple other chemokines from the Th2 pathway.15 In vitro work has shown that Th2 cytokines, particularly IL-4 and IL-13, alone and in combination can inhibit the epidermal differentiation proteins filaggrin,16 loricrin,17 and involucrin.17 Further studies have shown that IL-4 and IL-13 also inhibit antimicrobial peptides (AMP);18 this is very relevant to AD, since the inhibition of AMP facilitates proliferation of Staphylococcus aureus19 and skin microbiome imbalance and also increases the risk of skin infections. There is growing evidence that the microbiome is abnormal in AD.20 The diversity of the microbiome is reduced in patients with AD, with a larger proportion of S. aureus, which exacerbates during flares and improves when treated successfully.20
Potential Role of Th2 Cytokines in Atopic Dermatitis
Th2 cytokines may be the missing link between the barrier and the immune abnormalities in AD. These cytokines are associated with increased S. aureus binding and colonisation,16,21 decreases in antimicrobial peptides,1 and inhibition of lipid synthesis.1 In addition, there are some systemic effects, such as the promotion of dendritic cell differentiation and activation, supporting the activation and survival of Th2 cells,21,22 activating B cells, and promoting IgE class switching.21,22
This has led to the paradigm shift currently underway in the understanding of the pathogenesis of AD (Figure 1). Cytokines perpetuate the disease from the non-lesional stage, in which there is noticeable subclinical inflammation, to a higher increase in acute disease and even higher in the chronic stage.23-25 The Th2 cytokines IL-4 and IL-13 inhibit antimicrobial peptides and barrier proteins. IL-31 starts the itch-scratch cycle, while IL-22 starts the hyperplasia process, and, together with IL-17, synergises to increase S100 proteins, which is perpetuated to cause chronic disease.23-25 This understanding is very relevant to many treatments now being developed for AD.
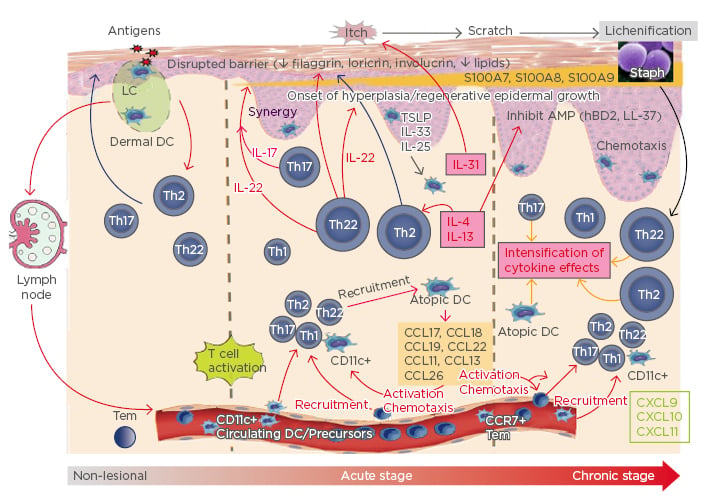
Figure 1: A paradigm shift in the pathogenesis of atopic dermatitis.
Understanding the potential role of Th2 cytokines as the mssing link between the barrier and immune abnormalities in AD has led to a paradigm shift in the understanding of the pathogenesis of AD.
AD: atopic dermatitis; AMP: antimicrobial peptide; CCL: chemokine ligand; CXCL: CXC chemokine ligand; DC: dendritic cell; hBD2: human β-defensin-2; LC: Langerhans cell; TSLP: thymic stromal lymphopoietin.
Adapted from Noda et al.,23 Gandhi et al.,24 and Wynn25
Atopic Dermatitis is Emerging as a Systemic Disease
Another important development is the understanding that AD is emerging as a systemic disease. The role of systemic inflammation is well established for psoriasis,4 but was less clear in AD until recent studies have showed increases in activated T cells, circulatory cytokines, and B cells compared with healthy volunteers and psoriasis patients.26 The recent meta-analysis-derived robust AD transcriptome showed that atherosclerosis signalling is very important in AD but not in psoriasis.27 This is supported by research showing an association between adult AD, cardiovascular disease, and increased heart attacks in several population-based studies.28
The systemic nature of AD leads to the comorbidities that are common with the condition, including allergic comorbidities such as asthma and allergic rhinitis, cardiovascular disease, and infectious diseases.29,30 Prof Guttman-Yassky emphasised the need for systemic treatment approaches for patients with moderate-to-severe AD.30
Evidence for Contribution of the Th2 Immune Axis to Atopic Dermatitis
Evidence for the immune hypothesis and contribution of the Th2 immune axis to AD is being tested with targeted Th2 treatments in AD patients. This has been made possible by the development of dupilumab, a fully human IL-4Rα monoclonal antibody that potently inhibits both IL-4 and IL-13 signalling.
A 4-week study of weekly injections of dupilumab (75 mg, 150 mg, and 300 mg, noting that the only licensed dose for dupilumab in moderate-to-severe AD is 300 mg every other week) or placebo proved the immune hypothesis.8,30 The study of 67 patients showed a clear dose response by Week 2 that continued to Week 4; 71.4% of patients on high-dose dupilumab achieved Eczema Area Severity Index (EASI) 50 (50% of disease was resolved). Importantly, there were no differences in responses based on filaggrin mutation status or IgE levels.8,30 A biopsy sub-study in 18 patients (10 with matching pre and post-treatment biopsies) demonstrated clear-cut results. A heat map of mRNA expression clearly showed that dupilumab (300 mg every other week) downregulated multiple immune markers, including many involved in the Th2 pathway, such as chemokine ligand 17 and 18, and progressively reversed the AD transcriptome.31 Modulation beyond Th2 pathways also occurred, notably with the Th17 and Th22 pathways.
The study did not include histochemistry but keratin 16 (K16) was measured as a marker of epidermal proliferation and hyperplasia. Results showed a dose response, with a 10-fold reduction in K16 with dupilumab 300 mg and a two-fold reduction with the 150 mg dose, in contrast to a two-fold increase with placebo.30,32
A recently published study of 54 patients with moderate-to-severe AD showed a major shift towards a non-lesional profile with dupilumab by Week 4, with a further shift at Week 16.31 Patients with lower EASI scores at baseline resolved by Week 4. This took longer for patients with higher EASI scores but by Week 16, lesional skin looked very similar to non-lesional skin. Further results showed that dupilumab reversed dysregulation of the AD transcriptome while also reversing the lesional phenotype towards the non-lesional phenotype by Week 16.31
Results also showed epidermal deficiencies in AD were reversed by dupilumab. K16 expression was completely turned off and filaggrin expression was normalised with dupilumab but not with placebo. Epidermal thickness was reduced by >40% with dupilumab, while there was no change in the placebo group.31 Patients treated with dupilumab reported major reductions in symptoms and an improvement in quality of life.
Prof Guttman-Yassky concluded that dupilumab impacts both the inflammation and barrier dysfunction of AD, establishing the Th2 axis, specifically IL-4 and IL-13, as the pathogenic driver in AD and confirming AD as a reversible, systemic, immune-driven disease.23-25
Clinical Management of the Patient With Moderate-to-Severe Atopic Dermatitis
Professor Thomas Bieber
AD is a highly complex disease requiring objective clinical assessment in addition to evaluation of the impact on the patient based on their subjective evaluation of signs and symptoms and the impact on quality of life measured by validated PRO. All AD patients experience pruritus; many have frequent and intense itch33 that has a huge impact on quality of life. They may also have atopic comorbidities, including allergic rhinitis and asthma,33 and bacterial, viral, and fungal skin infections.34 AD adversely affects daily activities, social functioning, and life decisions, impairing quality of life.35 Sleep disturbance is a major factor that affects patients’ performance in everyday life, at school, and at work.33
The ideal assessment of a patient with AD should include the use of an objective tool together with a tool for the patient to assess the impact of AD on their lives. The most commonly used tools measuring objective clinical signs include:
- EASI:36,37 maximum score 72 (<7=mild, >7–21=moderate, and >21=severe)
- SCORing Atopic Dermatitis (SCORAD):38 maximum score 103 (<25 mild, 25–50=moderate, and >50=severe)
- Body surface area (BSA)
- Investigator’s Global Assessment (IGA):39,40 maximum score 4 (0=clear, 1=almost clear, 2=mild, 3=moderate, and 4=severe)
The most commonly used tools based on PRO for AD include:
- Patient-Oriented Eczema Measure (POEM):41 maximum score 28 (0–2=clear or almost clear, 3–7=mild, 8–16=moderate, 17–24=severe, and 25–28=very severe)
- Hospital Anxiety and Depression Scale (HADS):42,43 maximum score 42 (anxiety or depression, 0–21 points each; >8 indicates symptoms of anxiety or depression)
- Patient-Oriented SCORAD (PO-SCORAD), which strongly correlates to SCORAD
- Dermatology Life Quality Index (DLQI):44,45 maximum score 30 (0–1=no effect, 2–5=small effect, 6–10=moderate effect, 11–20=very large effect, and 21–30=extremely large effect)
In addition, patients can be assessed for pruritus46 and sleep47 (maximum score 10, with a higher score indicating worse symptoms in both assessments). These are typically evaluated on 10-point visual analogue scales but can also be assessed on a numerical rating scale (NRS).
Assessing the Patient’s Perspective
In practice, it is important to realise that a clinician’s objective evaluation of the severity of AD is only one aspect of assessment. No single score captures the diverse signs, symptoms, and quality of life impact of AD. Clinicians tend to focus on skin lesions and pruritus; however, other aspects are often more noticeable to the patient, including sleep (which may be assessed with SCORAD and POEM), mental health (with growing recognition of systemic involvement in AD, assessed in HADS), and quality of life (assessed with DLQI).
One single score does not reflect the whole disease burden and there can be major discrepancies between the objective evaluation by the physician and the patient’s perception of his or her disease. Prof Bieber gave the example of Mr Z, who was aged 45 years and had had AD for 11 years. His EASI score was 16.45 points (from a maximum of 72), which is classified as moderate AD, and his POEM was 14 (from a maximum of 28, also corresponding to moderate). Then he presented Mrs Y, who had a similar EASI (18; i.e., moderate), but a POEM of 28 (maximum possible score, corresponding to a very severe disease burden). He said that a doctor may have considered that this patient had moderate AD based on the EASI score but the POEM showed Mrs Y was experiencing a substantial impact on quality of life.
When consulting patients with AD, Prof Bieber recommended that clinicians should ideally record their medical history and comorbidities and carry out an objective clinical assessment using a validated scoring system to evaluate the severity of AD in addition to collecting PRO. This information puts the clinician in a better position to educate the patient about AD and to make the right decision in terms of management and therapeutics, he suggested. The patient’s view, as assessed by PRO, is of increasing importance for management decisions and also as part of reimbursement decisions by payers.
Considering how assessment impacts treatment decisions, Prof Bieber described the case of Mr W, a 48-year-old man who had had AD for 8 years with target lesions representative of AD in all affected areas of the body. His EASI score was 48 (severe), BSA >50%, and POEM was 28 (very severe impact), indicating high severity and a need for systemic AD treatment.
Recent treatment guidelines from the European Dermatology Forum48 for AD in adults recommend proactive therapy with agents such as topical tacrolimus or PUVA combination treatment (psoralen and ultraviolet A) instead of immunosuppressive drugs in cases of moderate AD. Prof Bieber noted there are several challenges with immunosuppressive drugs in AD; they cause a range of adverse effects, including infections, and require frequent laboratory assessments.49 Discontinuation rates can be high. A retrospective study showed that >50% of AD patients discontinued azathioprine within 1 year.50 Nearly half (40%) of patients discontinuing azathioprine and 20% of those discontinuing enteric-coated mycophenolate sodium after 1 year did so due to side effects.50
The limited efficacies of drugs such as methotrexate and azathioprine mean that AD is not always well controlled.51 A study comparing methotrexate (20.0 mg/week) and azathioprine (2.2 mg/kg/day) showed that the mean SCORAD score was reduced by only 50% at 12 weeks,51 and remained at around 30%, which corresponds to moderate disease severity.
A further problem with systemic immunosuppressants is the risk of drug–drug interactions (DDI), which are becoming more likely with the ageing population and polypharmacy.52 The majority of moderate-to-severe AD patients are potentially unsuitable for systemic immunosuppressant therapy, primarily due to the risk of DDI, according to a large study.53 The study of >170,000 patients in a claims database found that 88% were unsuitable for systemic immunosuppressant therapy because of short-term DDI and 69% because of long-term DDI, while this treatment was contraindicated in 10% of patients.53
Most cases of cyclosporine, azathioprine, methotrexate, and mycophenolate mofetil use in AD are off-label. These drugs are broadly immunosuppressive and do not target the specific pathways involved in AD pathogenesis.1,49,54 Generally, they are used on a short-term basis because of potential safety risks and warnings; long-term use for disease control may be inadequate and is not recommended.49,55-60 Relapse after initial response may occur, for example, with cyclosporine, or rebound exacerbation of signs and symptoms on discontinuation, such as with oral corticosteroids.49,61 These problems highlight the need for effective and well-tolerated systemic therapies for moderate-to-severe AD.
Improving Patient-Reported Outcomes and Clinical Outcomes
Returning to the case of Mr W with severe AD, Prof Bieber explained he was treated for some time with methotrexate, but treatment had to be stopped because of side effects. He was then treated with dupilumab and the results illustrated the importance of using multiple assessment tools including PRO (Figure 2). The patient showed a substantial reduction in EASI score from 48.0 at baseline to 13.3 at Week 16 of dupilumab treatment. However, there was much greater improvement in the subjective assessment by the patient, with a reduction in POEM from 28 to 9 and a >90% reduction in other PRO, including DLQI, sleep, and pruritus. This demonstrated the improvement seen on the skin but an even greater improvement in quality of life and the patient’s perception of benefit.
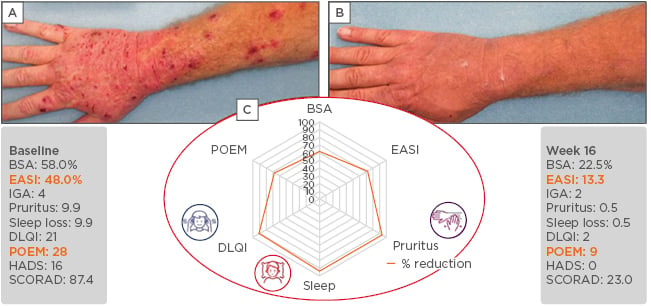
Figure 2: Mr W, 48 years of age with atopic dermatitis for 8 years. Before (A) and after (B) 16 weeks of treatment with dupilumab, and (C) the percentage reduction in the six key assessment scores.
The use of multiple assessment tools, including patient reported outcomes illustrate how single measures of assessment can underestimate the improvement in patient benefit.
BSA: body surface area; DLQI: Dermatology Life Quality Index; EASI: Eczema Area Severity Index; HADS: Hospital Anxiety and Depression Scale; IGA: Investigator’s Global Assessment; POEM: Patient-Oriented Eczema Measure.
Photos courtesy of Sanofi Genzyme and Regeneron. This is an individual case and not representative of results from all patients.
Challenges of Long-Term Therapy in Moderate-to-Severe Atopic Dermatitis
Professor Diamant Thaçi
AD is a challenging disease that persists for decades in many patients and has a substantial burden in some individuals, which highlights the need for effective and well-tolerated long-term systemic treatment. The immunosuppressants currently available are effective for the short-term treatment of AD but managing patients over the long term is challenging, mainly because of side effects.
Cyclosporine A (CsA) is approved for the long-term treatment of AD in many countries; however, the summary of product characteristics57 advises that once a satisfactory response is achieved, the dose should be reduced gradually and, if possible, cyclosporine should be discontinued. Guidelines argue against long-term treatment.48 The European Dermatology Forum guidelines caution that treatment should not exceed a 2-year continuous regimen.48 Duration should be guided by treatment efficacy and the tolerance and side effects of CsA make it unsuitable for long-term treatment of AD.48 The current European Academy of Dermatology and Venereology (EADV) guidelines make similar recommendations,54 leaving clinicians with the challenge of how to manage AD in the long-term.
Guidelines provide limited information on the long-term off-label use of immunosuppressants, such as methotrexate, azathioprine, and mycophenolate mofetil.48,49,54,62 They generally recommend their use after CsA, tapering the dose and discontinuing after a treatment effect has been achieved. A study investigating the median duration of use of immunosuppressants showed that it did not exceed 9.8 months in clinical practice.63 For CsA, the median duration of treatment was 8.5 months and 24% of patients with severe AD discontinued treatment due to adverse events, including serum creatinine increase (10%), hypertension (7%), and neurologic symptoms (6%).63
New treatments for moderate-to-severe AD must have long-term efficacy and safety, demonstrated in clinical trials and in daily practice, suggested Prof Thaçi. He considered that dupilumab provides an important advance in meeting this goal. Immunosuppressants have a broad immunosuppressive mechanism of action4,49 and do not specifically target the underlying inflammation that drives pathogenesis. In contrast, dupilumab is a targeted treatment4,9 that selectively inhibits IL-4 and IL-13 signalling, which are key cytokines driving the underlying persistent inflammation in AD.
In terms of safety, immunosuppressants are associated with potentially serious side effects,49,55-57 so their use is limited to a short period of time, while AD is a chronic disease. Serious adverse events include lymphomas and other malignancies, infections, hepatotoxicity, renal toxicity, bone marrow suppression, pulmonary toxicity, neurotoxicity, thromboembolic events, anaphylactic shock, Stevens–Johnson syndrome, and embryofetal toxicity.55-57 Clinical trial data have suggested that dupilumab has an acceptable safety/tolerability profile for long-term use when used concomitantly with topical therapy or as monotherapy.4,40,64-67 The most common adverse reactions were injection-site reactions, conjunctivitis, blepharitis, and oral herpes; <1% of patients experienced hypersensitivity reactions.4,40,64-67 However, hypersensitivity reactions (urticaria, rash, erythema nodosum, anaphylaxis, and serum sickness) have occurred after administration of dupilumab66 and treatment should be discontinued in the event of a hypersensitivity reaction.66
Long-Term Studies of Dupilumab in Patients With Moderate-To-Severe Atopic Dermatitis
Three studies have investigated dupilumab in patients with moderate-to-severe AD. LIBERTY AD SOLO-CONTINUE included 422 patients who were treated for 36 weeks plus a follow-up period of 12 weeks.40,68 CHRONOS was a 52-week study of dupilumab with concomitant topical corticosteroids in 740 patients with an EASI score ≥16 at baseline, including safety follow-up through Week 64.64 An open-label extension study included 1,491 patients who had previously participated in Phase I–III clinical studies of dupilumab, and involved patients treated on a once-a-week basis for up to 3 years, including patients continuously treated or retreated with dupilumab and patients undergoing dupilumab interrupted treatment. 69
Results were better than in the pivotal trials (SOLO1 and SOLO2).40 For example, in CHRONOS, 39% of patients treated with dupilumab once every 2 weeks plus topical corticosteroids achieved IGA 0/1 versus 12% of the placebo group at Week 16, and >60% of those given dupilumab achieved EASI 75 compared with 23% of the placebo group.64 At Week 52, the results were similar (Figure 3). Further analysis showed a sustained response to dupilumab in a high proportion of patients, with the response maintained at different timepoints until 52 weeks.70 These results suggest dupilumab provides an important advance in achieving long-term management of AD, suggested Prof Thaçi.
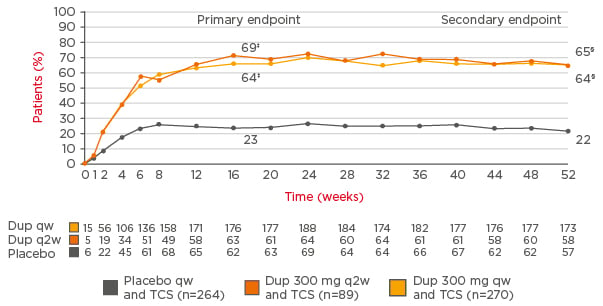
Figure 3: Sustained improvements in Eczema Area Severity Index 75 responder rates* in the CHRONOS study (concomitant topical corticosteroids for 52 weeks†).
Long-term follow-up of patients in the CHRONOS study shows Eczema Area Severity Index responder rates with dupilumab are sustained at 1 year.
*The only licensed dose of dupilumab in patients with moderate-to-severe AD is 300 mg once every 2 weeks.†Multiple imputation method with ANCOVA in which data after rescue treatment were set to missing and then missing data were imputed using multiple imputation, with treatment, randomised strata, and the corresponding baseline value of the endpoint included in the model.‡p<0.0001 (full analytical set). §p<0.0001 (full analytical set, Week 52).
AD: atopic dermatitis; Dup: dupilumab; q2w: every 2 weeks; qw: weekly; TCS: topical corticosteroids.
Adapted from Blauvelt et al.64
Controlling itch in AD over the long term is important, in addition to controlling inflammation. Steroids have limited efficacy in controlling itch; however, results from CHRONOS showed significant improvements in pruritus with dupilumab occurring as early as Week 2 of treatment and continuing through to Week 52, with 51% of patients achieving a Peak Pruritus NRS improvement ≥4 points.64 There were also sustained improvements in DLQI and POEM. Improvement in the symptoms of AD leads to improvement in quality of life, said Prof Thaçi.
The long-term impact of dupilumab was illustrated by the case of a 31-year-old patient with severe AD since childhood, with an IGA of 4, EASI 28.2, and itch 10/10, who was experiencing sleeplessness and fatigue. He had previously been treated with topical and systemic steroids, frequent ultraviolet B treatment, and CsA, which was stopped due to side effects and a lack of effectiveness. After 4 weeks of dupilumab, his skin lesions showed some improvement and itch also improved. Some skin lesions remained at 16 weeks; however, improvements continued for 3 years, demonstrating the value of long-term treatment with dupilumab.
Incorporating Long-Term Treatment into Routine Clinical Practice
The open-label extension Phase III study in adults with moderate-to-severe AD involved 1,491 patients who had participated in previous dupilumab studies, including 399 who completed Week 52 analyses.69 The primary endpoints were incidence and duration-adjusted rates of adverse events, as well as EASI 75 at Week 52. Results showed that nearly half of the patients (48.6%) had clear or almost clear skin (IGA score 0/1) and approximately three-quarters (75.4%) had at least 75% improvement in EASI score at 52 weeks. The Peak Pruritus NRS decreased from an average of 8 to 2.29 and the DLQI score improved to an average of 2.9.69
In a subset of patients who had interrupted treatment for >13 weeks, the study showed that treatment interruption did not affect dupilumab long-term efficacy or tolerability. The Phase III open-label study showed that dupilumab maintained efficacy after 52 weeks of treatment at a level comparable to that seen in the parent trial, with mean EASI scores maintained over time. In addition, treatment interruption did not impact the long-term tolerability of dupilumab.71 However, retreated patients had a slightly higher level of antidrug antibodies, similar to that seen with other biologics.
Illustrating the long-term treatment of severe AD with dupilumab, Prof Thaçi described a particularly challenging case of a 58-year-old female patient with chronic, recurrent AD that had persisted for 40 years. Her disease severity in September 2013 was IGA 4, EASI score 33.3, BSA 43%, and pruritus 9/10 NRS. Previous treatment included many courses of oral corticosteroids and a diverse range of potent topical steroids. He reported that long-term treatment with dupilumab achieved dramatic improvement after 5 years, with major reductions in active skin lesions and itch.







