Speakers: Kenneth B. Gordon,1 Kristian Reich,2 Peter C. Taylor3
1. Department of Dermatology, Froedtert Hospital and the Medical College of Wisconsin, Milwaukee, Wisconsin, USA
2. Translational Research in Inflammatory Skin Diseases, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
3. Botnar Research Centre, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford and St Peter’s College, Oxford, UK
Disclosure: Prof Gordon has received consultation fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly, Janssen, Novartis, and Pfizer; and has received research grants from AbbVie, Amgen, Celgene, and Janssen. Prof Reich has been an advisor and/or paid speaker for and/or participated in clinical trials for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Centocor, Covagen, Dermira, Eli Lilly, Forward Pharma, Fresenius, Galapagos, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Medac, Miltenyi Biotec, MSD, Novartis, ocean pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and Xenoport. Prof Taylor has received consultation fees from AbbVie, Biogen, Bristol Myers Squibb, Eli Lilly, Fresenius, Galapagos, Gilead, GlaxoSmithKline, Janssen, Nordic Pharma, Pfizer, Roche, Sanofi, and UCB; and has received research grants from Celgene, Galapagos, Gilead, and Eli Lilly.
Acknowledgements: Medical writing assistance was provided by Dr Megan Breuer, Excerpta Medica, Amsterdam, the Netherlands.
Support: Both the symposium and the publication of this article were funded by Janssen. The views and opinions expressed are those of the authors and are not necessarily those of Janssen.
Citation: EMJ Dermatol. 2020;8[1]:24-31.
Meeting Summary
The symposium, “The Other Side of the Moon: A Clinical Dialogue on the IL-23 Pathway”, took place during the European Academy of Dermatology and Venereology (EADV) Virtual Congress on 29th October 2020. Distinguished experts Prof Gordon, Prof Reich, and Prof Taylor highlighted how the IL-23 pathway has emerged as a promising target in the management of psoriatic diseases. The expert faculty provided virtual attendees with updates on the newest developments from both dermatological and rheumatological perspectives, offering invaluable insights into psoriasis and psoriatic arthritis (PsA), as well as clinical guidance on managing these conditions in everyday practice.
Around the World in 15 Minutes: Recent Insights into IL-23 in Psoriasis
Professor Kristian Reich
Prof Reich began by highlighting how insights into the pathophysiology of psoriasis have led to the development and expansion of cytokine-targeted therapies, beginning with anti-TNF therapies in the early 2000s, and evolving to therapies targeting the IL-12, IL-17, and IL-23 pathways in the past decade. Now, with the introduction of treatments that block the p19 subunit of IL-23, therapies are available that solely block IL-23.
Biopsies from psoriatic skin have revealed that T cells are activated by dendritic cells, which requires the presentation of antigens to T cells, costimulatory signals given by cell-surface molecules, and signals received from ‘educational cytokines’ released by dendritic cells to determine functional Th cell subsets.1,2 The current model of psoriasis immunopathogenesis emphasises the role of the IL-23 pathway in the production of IL-17 by T cells, thus inducing the hyperproliferation of keratinocytes and a psoriatic phenotype. Prof Reich also emphasised the role of keratinocytes in the production of a multitude of cytokines, and the interplay between keratinocytes and T cells, representing a cycle of disease perpetuation.3 The evolution of the treatment landscape has resulted in a sharper focus on IL-12/23 inhibition, with emphasis on the inhibition of IL-23 and the p40 subunit (IL-23p40). Results of trials with briakinumab and ustekinumab have revealed that these therapies result in swift improvements in Psoriasis Area and Severity Index (PASI) 75, 90, and 100 scores after 12 weeks of treatment.4-6 Further examination has revealed a critical role of IL-23, as seen in a study of IL-23 subunit p19 and p40 expression in paired samples of uninvolved and lesioned skin from patients with psoriasis.7
The breakthroughs in the past decade have led to a re-examination of the psoriasis disease model, with a greater focus on the pathways associated with innate immunity, synergistic proinflammatory effects, and keratinocyte proliferation.3 The results of the Phase III UltIMMa-1 and -2, IMMvent, and IMMhance trials have shown that treatment with risankizumab resulted in significant improvements in static Physician’s Global Assessment (sPGA) 0/1 and PASI 90 scores at Week 16, compared with placebo, adalimumab, and ustekinumab.8-10 Furthermore, a network meta-analysis that assessed the probability of achieving PASI 90 or 100 scores at the primary endpoint at Weeks 10–16 of treatment, and at the end of the maintenance period at Weeks 44–60, revealed that a significant proportion of patients achieved these scores when receiving treatment with ixekizumab, brodalumab, risankizumab, and guselkumab at both time points, and additionally, secukinumab during the maintenance period.11 Treatment with guselkumab has also been shown to result in long-term efficacy, with 86.2% of patients (as observed) maintaining a response over 5 years.12
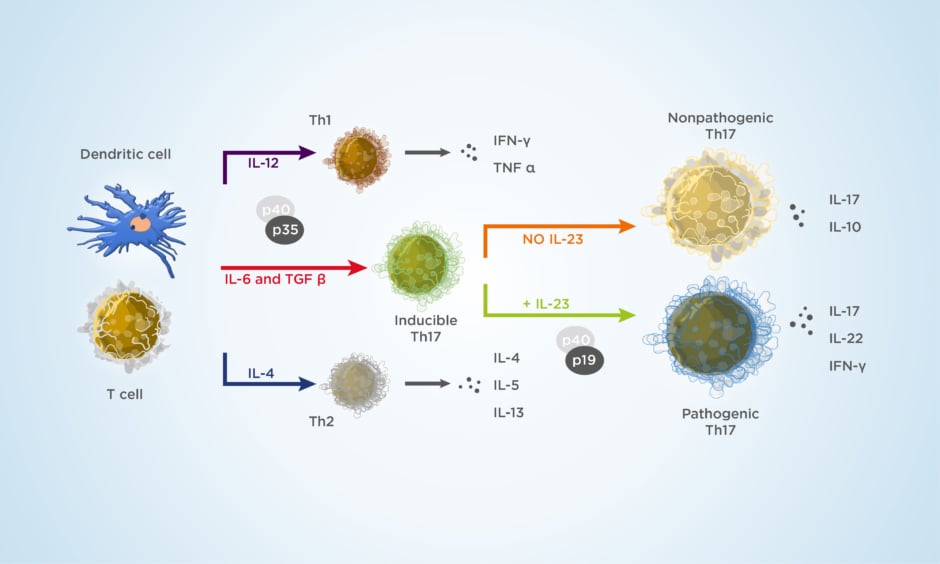
Insights from patients with a genetic deficiency of IL-17 point to a role of the cytokine in protecting from Candida spp. infection.13 Interestingly, targeting IL-23 appears to leave the physiological role of the IL-17 pathway intact (Figure 1).14,15
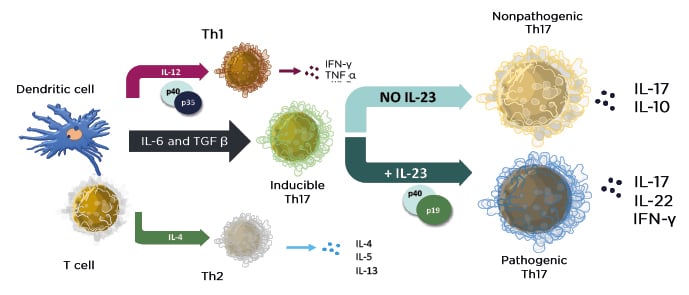
Figure 1: The cytokine environment regulates lymphocyte differentiation into functional subsets.
Adapted from Zhu J et al.15
Results from the IMMhance and VOYAGE 2 trials with risankizumab and guselkumab, respectively, showed that treatment response can be maintained after treatment withdrawal, indicating a possible ‘disease reprogramming’ in patients with sustained responses.16,17 Parameters predictive of the maintenance of PASI 90 responses to guselkumab following treatment withdrawal included shorter disease duration and lower BMI at baseline, and PASI 100 and Investigator’s Global Assessment (IGA) 0 responses at Week 28 of treatment.18 Underlying the maintenance of response are tissue-resident memory (Trm) cells, which remain elevated in clinically nonactive psoriatic lesions, produce IL-17, and may drive disease recurrence.19
Biopsy data from the ECLIPSE trial were used to examine T-cell frequency in psoriatic lesions and skin of patients treated with guselkumab or secukinumab to further differentiate between the effects of IL-23 and IL-17 blockade on psoriasis outcomes. The results showed that, at Week 24 of treatment, significant differences in the frequency of cluster of differentiation (CD)8+ Trm cells within CD3 T cells were observed between treatments, with reduced numbers of Trm cells in patients who received guselkumab treatment, compared with secukinumab.20
Safety analyses of biologic therapies for psoriasis have revealed that there were low absolute numbers of safety events of interest, and no target-specific safety observations for IL-23 inhibitors (Reich, personal communication).
Prof Reich concluded that IL-23 is a key mediator in psoriatic skin inflammation, though the exact role of IL-23 in PsA domains (for example, enthesitis) is unclear. Inhibition of IL-23 appeared to be very safe and induced a high level of stable and sustainable clinical response in most patients with psoriasis.
The Translational Journey of IL-23 Pathway Inhibitors in Psoriasis
Professor Kenneth B. Gordon
There is much to be learnt from IL-23 inhibitors in the treatment of psoriasis, including information about the long-term efficacy of these treatments and use in special populations, as well as information regarding treatment withdrawal and retreatment, patient-reported outcomes (PRO), and long-term safety implications. In order to better understand the efficacy of IL-23p19 inhibition on psoriasis outcomes, the VOYAGE 1 and VOYAGE 2 trials examined the impact of treatment with guselkumab 100 mg, given every 8 weeks (q8w), compared with adalimumab, given at 40 mg every 2 weeks, on PASI outcomes in patients with mild-to-moderate psoriasis.21,22 In VOYAGE 2, patients who received placebo from Weeks 0 to 16 crossed over to guselkumab 100 mg at Week 16.21,22
Results from the VOYAGE 1, UltIMMa-1 and -2, and reSURFACE 2 trials demonstrated the high clinical efficacy of IL-23p19 inhibitors, and high proportions of patients achieved PASI 90 responses after treatment with guselkumab, risankizumab, or tildrakizumab.21,23,24 High levels of PASI responses were also maintained through Week 252 and Week 148 with guselkumab and tildrakizumab treatment, respectively.12,25 A recent post hoc analysis showed that 88 of 494 (17.8%) patients treated with guselkumab from Week 0 or 16 in the VOYAGE 1 trial maintained PASI 0 scores at all visits for a period of 3 years.26
Furthermore, PASI 90 responses were maintained in 11.5% of patients 52 weeks after withdrawal from guselkumab, and response was regained in up to 85.7% of patients after treatment reinitiation.17 These results further underscored the hypothesis that complete elimination of Trm cells may be required to achieve a long-term treatment response. Similarly, PASI 90 responses were maintained in sPGA 0/1 responders to risankizumab after withdrawal.27 Pooled data from the VOYAGE 1 and 2 trials showed that patients who received guselkumab treatment showed comparable IGA 1/0 responses to treatment, regardless of body weight, while patients with higher body weights who received adalimumab showed decreased IGA 1/0 responses.28
The PRO tended to mirror the clinical outcome results; patients who received guselkumab and adalimumab reported significant improvements in the Dermatology Life Quality Index (DLQI), achieving 0/1 scores at Weeks 8 and 16 of treatment, compared with placebo (p<0.001). At Week 24 of treatment, 58.9% of patients who received guselkumab achieved DLQI 0/1 scores, compared with 40.2% of patients who received adalimumab (p<0.001).29 Similarly, in the reSURFACE 1 and 2 trials, a numerically higher proportion of patients who received tildrakizumab with high PASI scores (90–100) achieved DLQI 0/1 scores, compared with patients with PASI scores 75–89 or 50–74.30 In the UltIMMa-1 and -2 trials, a significant proportion of patients who received risankizumab achieved DLQI 0/1 scores, compared with ustekinumab (p<0.0001).8 Prof Gordon emphasised that psoriasis is a disease that impacts quality of life and highlighted how the elimination of the disease impacts the patient’s daily life as a major treatment goal. The Psoriasis Symptoms and Signs Diary (PSSD) is an alternative PRO assessment tool, in which patients can assess and record the severity of their psoriasis symptoms and signs using a scale of 1 to 10. The assessed symptoms include itch, burning, stinging, skin tightness, and pain; the assessed signs include skin dryness, cracking, scaling, shedding or flaking, redness, and bleeding. Scores can range from 0 to 100, with a score of 0 representing symptom- and sign-free status. Notably, in a combined analysis of the VOYAGE 1 and 2 studies, a higher proportion of guselkumab-treated patients achieved a PSSD symptom or sign score of 0 at Week 24, compared with adalimumab-treated patients (Figure 2).31 Of the patients who received continuous guselkumab, 94.4% of patients with PSSD symptom scores of 0 achieved DLQI 0/1 scores at Week 24.29 Furthermore, 79.4% of patients who achieved PASI 100 also achieved DLQI 0/1 at Week 24, compared with only 65.8% of patients who received adalimumab. For patients achieving PASI <100, there was no notable difference between the two treatments.29 Data from a transcriptomic study suggested that the patients who achieved clearance (PASI 100) of their skin symptoms with guselkumab also demonstrated a normalisation of previously dysregulated genes in cleared lesional skin, whereas biopsies from cleared lesional skin of adalimumab-treated patients showed that the majority of genes had persistent dysregulated expression. This may partly account for the lower percentage of DLQI 0/1 scores in patients treated with adalimumab.31
Figure 2: Proportions of patients achieving A) symptom-free or B) sign-free status at Week 24.
*p<0.05
†p<0.01
Combined analysis of PASI 100 responders from VOYAGE 1 and 2 studies.
ADA: adalimumab; GUS: guselkumab; PASI: Psoriasis Area and Severity Index.
Adapted from Blauvelt A et al.31
Safety analyses of IL-23 inhibitors have revealed no new safety signals; a study examining tildrakizumab revealed no novel or unexpected safety signals through Week 148 of treatment,25 while studies with guselkumab showed no new safety signals and none that increased with exposure over time.32
Prof Gordon concluded that clinical trial results have demonstrated consistent, high-level efficacy, and good DLQI responses that can be well maintained for years after treatment initiation. The VOYAGE trials have identified specific mechanisms driving the re-emergence of psoriasis after treatment cessation, creating unique and exciting possibilities for furthering the understanding of psoriasis signs and symptoms during treatment.
Transferring Orbits: IL-23 in Psoriatic Arthritis
Professor Peter C. Taylor
The spectrum of spondyloarthritis (SpA) encompasses both axial and peripheral manifestations, including nonradiographic axial SpA, ankylosing spondylitis, and PsA.33 Several clinical trials have examined possible treatments for PsA, including TNF, IL-17, and IL-23 inhibitors.34 In particular, IL-23 seems to be the main unifying factor in SpA; IL-23 sensitivity is associated with psoriasis and inflammatory bowel disease, and IL-23 overproduction is associated with SpA development.35 Research has also shown that IL-23-responsive entheseal cells drive SpA, and that IL-23 and resident T cells promote enthesitis and osteoproliferation.36,37
Results from recent clinical trials have shown that several treatments, including TNF, IL-17, IL-12/23, and IL-23 inhibitors, all have an impact on American College of Rheumatology 20% (ACR 20) responses in patients with PsA.38-41 However, a shifting focus on the p19 subunit of IL-23 has revealed that this specific inhibition resulted in significant proportions of patients achieving ACR 20 scores at Week 24 of treatment with guselkumab 100 mg every 4 weeks (q4w) and q8w, compared with placebo, in the DISCOVER-1 and -2 trials (p<0.0001 for both dosages in DISCOVER-2; p<0.001 for both dosages in DISCOVER-1) (Table 1).41,42 Furthermore, in DISCOVER-1, significant proportions of patients who received either dosage of guselkumab also achieved ACR 50 scores at Week 24 of treatment, compared with placebo (p<0.001 for both dosages). A significant proportion of patients who received the guselkumab q4w dosage also achieved ACR 70 scores at Week 24, compared with placebo (p<0.001) (Table 1).42
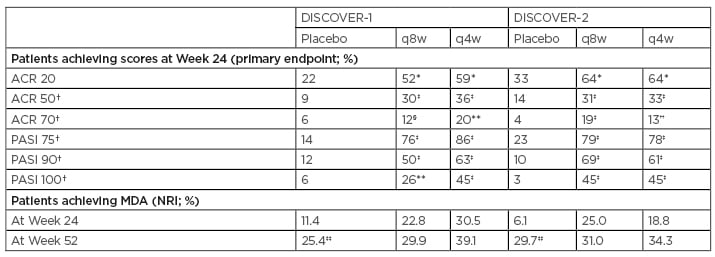
Table 1: DISCOVER-1 and DISCOVER-2 joint (ACR) and skin (PASI) outcomes at Week 24 of treatment, and minimal disease activity outcomes at Weeks 24 and 52 of treatment.41-44
*p<0.0001 (USA procedure adjusted)
†ACR 50 and 70 scores and PASI 75, 90, and 100 scores were not multiplicity controlled in the USA-specific procedure
‡p<0.0001 (unadjusted)
§p=0.0069 (unadjusted)
**p=0.0005 (unadjusted)
††p=0.0004 (unadjusted)
‡‡Patients who received placebo crossed over to guselkumab 100 mg q4w at Week 24.
ACR: American College of Rheumatology; MDA: minimal disease activity; NRI: nonresponder imputation; PASI: Psoriasis Area Severity Index; q4w: every 4 weeks; q8w: every 8 weeks.
The q4w and q8w dosages of guselkumab treatment also resulted in significant improvements in PASI 75, 90, and 100 scores, compared with placebo, at Week 24 of treatment in both the DISCOVER-1 and -2 trials.41,42 The proportions of patients with minimal disease activity at Week 24 were higher with guselkumab than with placebo in the DISCOVER-1 and -2 trials, and response rates continued to rise through 1 year (Table 1).43,44
An analysis of treatment-emergent adverse events through Week 52 in DISCOVER-2 revealed no opportunistic infections or active tuberculosis, no inflammatory bowel disease, and no deaths associated with guselkumab treatment;41,43 this was similar to the safety findings through the end of DISCOVER-1.42,44
Studies with several dosages of tildrakizumab have revealed that patients who received treatment also showed increased ACR 20 response rates, with significant improvements in PASI scores at Week 52 of treatment in patients with PsA.45,46 Similarly, there were favourable ACR 20, 50, and 70 responses with risankizumab, compared with placebo, in a Phase II open-label extension study in patients with PsA.47 In contrast to the effects in PsA, IL-23 inhibition failed to meet its primary endpoint in a study evaluating the efficacy of risankizumab in patients with ankylosing spondylitis, with no observed improvements in Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) or Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores, compared with placebo, at Week 12 of treatment.48 However, pooled analyses from the DISCOVER-1 and -2 studies in a subgroup of patients with PsA with axial involvement revealed that treatment with guselkumab 100 mg q4w and q8w resulted in greater improvements in BASDAI scores (p<0.001), and significant improvements in ASDAS-related endpoints (p<0.05), compared with placebo, at Week 24 of treatment.49
Prof Taylor concluded that PsA is a progressive disease that is associated with significant disability. The diverse clinical characteristics associated with PsA have represented several challenges regarding therapies, though recent advances in the understanding of the pathobiology of PsA have contributed to several therapeutic treatment options. Recent clinical trials have validated the IL-23 pathway as a therapeutic target in psoriasis and PsA, and treatments with IL-23 inhibitors, such as guselkumab, tildrakizumab, and risankizumab, have led to improved outcomes for patients with PsA.
Conclusions
The understanding of psoriasis pathophysiology has increased substantially in past years, allowing for the development of targeted therapies, such as IL-23p19 inhibitors. As such, it may be necessary to raise the bar regarding psoriasis treatment goals as achieving PASI 90 and PASI 100 scores have become attainable for more patients with the new therapies. Emerging evidence has underscored the need to eradicate Trm cells, which remain elevated in clinically nonactive psoriatic lesions and which may drive disease recurrence.
Recent studies have illuminated the role of PRO in assessing psoriasis management, emphasising the importance of quality-of-life scores and patient-reported improvements in psoriasis signs and symptoms. Furthermore, recent ground-breaking advances in the understanding of the pathobiology of PsA have contributed to several therapeutic treatment options. Treatment with IL-23 inhibitors, such as guselkumab, tildrakizumab, and risankizumab, has led to improved outcomes for patients with PsA, including improvements in ACR and minimal disease activity scores. Guselkumab treatment, in particular, led to enthesitis resolution, as well as improvements in ASDAS-CRP and BASDAI scores. Safety analyses for IL-23 inhibitors have revealed no new safety signals. Furthermore, no opportunistic infections or active tuberculosis, inflammatory bowel disease, or deaths were associated with long-term guselkumab treatment.
This symposium underlined the growing importance of the IL-23 pathway in the management of psoriatic diseases. The latest developments from dermatological and rheumatological studies highlighted the key role this pathway plays in the management of psoriatic disease conditions in everyday practice.
Date of preparation: November 2020
CP-192493