Meeting Summary
Prof Girolomoni provided an overview of psoriasis, considering how patients are frequently affected by other comorbidities. Cost, he explained, can be a constraint for optimal anti-tumour necrosis factor (TNF) treatment, with biosimilars representing an important opportunity for providing more patients with effective therapy. Data from X-ray crystallography studies, neutralisation studies, and clinical trials were presented, demonstrating that biosimilars have comparable efficacy to reference treatments.
Prof Sattar explained how to define the overall cardiovascular disease (CVD) risk score in psoriasis; the standard risk score should be multiplied by 1.5 for patients with young onset or more severe disease. Throughout the presentation he stressed that all CVD risk factors need to be taken into consideration. Just because someone has severe psoriasis does not mean they are necessarily at high risk of CVD, and just because someone has mild psoriasis does not mean they are at low risk. In the second part of his talk, Prof Sattar reviewed evidence suggesting that psoriasis and obesity are interlinked, and discussed benefits of weight loss.
Dr Behrens considered the hypotheses for psoriatic arthritis (PsA) genetic predisposition in patients with psoriasis. He reviewed data suggesting that psoriasis and PsA are different diseases, with psoriasis acting as a trigger for PsA. Dr Behrens went on to discuss predictors of PsA in patients with psoriasis and the importance of individualising treatment to phenotype.
Dr Gecse reviewed the aetiology, disease course, prognostic factors, and characteristics of inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC). She explained how the prevalence of CD and UC is four-times higher in patients with psoriasis versus the general population, with the highest rates occurring in patients with both psoriasis and PsA. She went on to present studies showing how interleukin (IL)-17 inhibitors, which show promising effects in psoriasis, worsened in IBD.
Introduction
Professor Giampiero Girolomoni
Psoriasis is a common disease affecting 3% of the population,1 said Prof Girolomoni. Around 10–20% of patients with psoriasis need systemic treatment2 and 5–30% have PsA.3,4 Psoriasis is a life-long disease with a chronic relapsing course, requiring long-term therapy for most patients.5 Biological treatments are associated with high costs which limits optimal treatment for many patients.6 Psoriasis, said Prof Girolomoni, affects all aspects of patient life including emotional,7 financial,8 work,9 and leisure.10
Patients with psoriasis frequently have associated disorders, which may be due to common pathogenesis resulting from shared genetic loci and cytokines (PsA and CD)11-13 or result from chronic inflammation (CVD, metabolic syndrome, and Parkinson’s disease).11,12,14-16 Finally, psoriasis has a psychosocial impact, and may result in depression, anxiety, smoking, and alcoholism.11,12
Current Treatment Landscape in Moderate-to-Severe Psoriasis
Professor Giampiero Girolomoni
Many factors influence psoriasis severity, said Prof Girolomoni, including body surface area involvement, erythema, infiltration and scaling, lesions in sensitive areas, impact on quality of life, non-response to treatments, disease activity, frequency, and severity of relapses.17 Such factors can be perceived differently by patients and doctors. One study, involving 2,513 patients and 391 dermatologists, showed patients believed itching to be the most important factor contributing to psoriasis severity (43%), while dermatologists said location and size of skin lesions (53%).18,19
With regard to psoriasis severity, it is important to take comorbidities into consideration. Severe psoriasis is an independent risk factor for metabolic comorbidities such as obesity, metabolic syndrome, non-alcoholic fatty liver disease (NAFLD), hypertension, dyslipidaemia, hyperuricaemia, and diabetes. The nature of associations is not well understood, but may be due to a common gene(s) and/or the effect of persistent severe skin inflammation. One study showed psoriasis shares predisposing genes with obesity, rheumatoid arthritis (RA), Type 2 diabetes mellitus, and Alzheimer’s disease.20
The Italian guidelines on systemic treatments for moderate-to-severe psoriasis recommend a holistic treatment approach taking into consideration disease (severity and frequency of relapses), the patient (contraindications, likelihood of adherence), and treatment (short and long-term effectiveness, practicability, flexibility, safety, tolerability).17
Factors in choice of biological treatment include overall efficacy, need for rapid response, and presence of concomitant diseases benefitting from the same treatment;17 however, the most common reason for not initiating biological therapy, said Prof Girolomoni, was cost. This view is supported by a recent survey where 48.8% of dermatologists and 45.9% of rheumatologists responded that cost was the main reason for not initiating biological therapy.6
Biosimilars, said Prof Girolomoni, have a place to ensure early, optimal, and equal access to anti-TNF treatment in psoriasis.21 Biosimilars can provide earlier access to effective treatment options and have the potential to improve treatment continuity and reduce regional variability in access across Europe. A recent paper provided an overview of the current status of anti-TNF biosimilars, showing the number of biosimilars that have been registered and the number of agents currently investigated in preclinical and the number clinical trials (Figure 1). Although two etanercept and six adalimumab biosimilars have been tested in psoriasis, no infliximab biosimilars have been tested in these patients.22
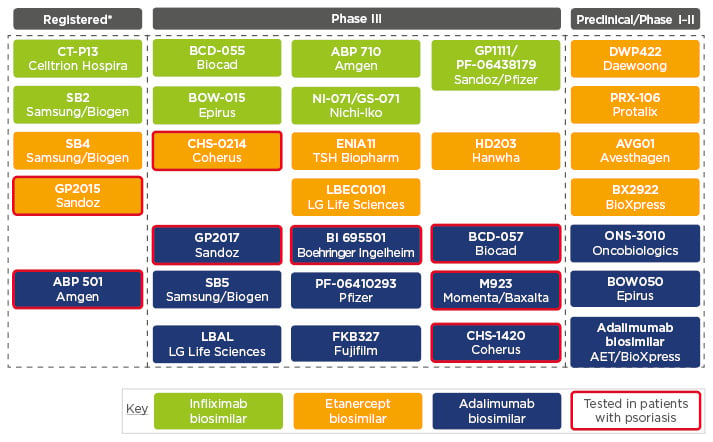
Figure 1: Principal anti-tumour necrosis factor biosimilars available or in development.
*Authorised by the European Medicines Agency (EMA), current status as of July 2017.
Adapted from Braun and Kay.22
A Phase III study comparing the adalimumab biosimilar ABP 501 with reference adalimumab showed that at Week 16, the mean Psoriasis Area Severity Index (PASI) percent improvement was comparable between the two groups.23
Several studies have shown that biosimilar and reference medicines have similar levels of efficacy and comparable safety, said Prof Girolomoni. X-ray crystallography analysis of Sandoz’s etanercept biosimilar GP2015 found that the higher order structure of GP2015 and reference etanercept were indistinguishable.24 In addition, similar protein content (essential for clinical efficacy of a biologic) and TNF binding and neutralisation were comparable between GP2015 and reference etanercept sourced from the European Union (EU) and the USA.24 The EGALITY study25 showed comparable efficacy, safety and immunogenicity between GP2015 and reference etanercept over 52 weeks in patients with psoriasis; switching between GP2015 and reference etanercept multiple times did not impact on efficacy and safety. Furthermore, the primary endpoint for equivalence, comparable PASI 75 response rates at week 12, was met. (See Figure 2 for multiple-switch study design).
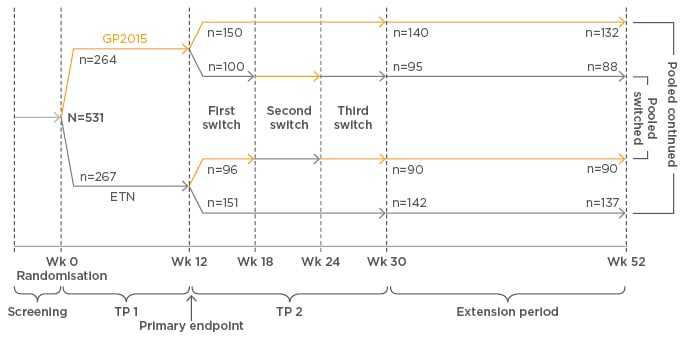
Figure 2: Bioequivalence of GP2015 was confirmed in patients with psoriasis in a multi-switch study (EGALITY).
ETN: reference etanercept; TP: treatment period; Wk: week.
Adapted from Griffiths.25
The EGALITY study2 showed comparable efficacy, safety, and immunogenicity (note: bioequivalence was confirmed at week 12, primary endpoint) of GP2015 and reference etanercept over 52 weeks in patients with psoriasis; switching between GP2015 and reference etanercept multiple times did not impact on efficacy and safety. Furthermore, the primary efficacy endpoint for equivalence, comparable PASI 75 response rates at week 12, was met (see Figure 2 for multiple-switch study design).
Cardiometabolic Risks in Psoriasis and Psoriatic Arthritis
Professor Naveed Sattar
Studies indicate patients with psoriasis are at increased risk of CVD, said Prof Sattar;26 but risk is only meaningfully increased in a subgroup, usual risk factors matter. In a prospective population cohort study of 556,995 controls, 127,139 patients with mild psoriasis, and 3,837 with severe psoriasis, relative risk (RR) for myocardial infarction (MI) was higher in younger patients.27
For 30-year-old patients, the adjusted RR for having a MI was 1.29 for mild disease, versus 3.10 for severe disease; while for a 60-year-old the adjusted RR for MI was 1.08 for mild psoriasis, versus 1.36 for severe psoriasis. Severe early-onset psoriasis is the group at the highest risk. The study cannot be considered definitive in terms of assessing independent risk, said Prof Sattar, since it did not fully adjust for residual confounding factors. Nevertheless, if risk is increased meaningfully, it is in younger patients with severe psoriasis.
In their CVD risk calculator, the Joint British Societies for the Prevention of Cardiovascular Disease use a 1.4 multiplication factor for patients with RA, after adjusting for other predictors.28 Similarly, EULAR guidelines recommend CVD risk prediction models are adapted for patients with RA and PsA using a 1.5 multiplication factor.29 The same approach can be undertaken for severe, early-onset psoriasis.
Two Case Studies
- Patient A: a 50-year-old man with no other conditions, BMI 23, cholesterol 6.5 mmol/L, systolic blood pressure 146 mmHg, who smoked 30 cigarettes per day, and had a family history of CVD.
- Patient B: a 50-year-old man with severe psoriasis for 38 years, BMI 34, cholesterol 4.8 mmol/L, systolic blood pressure 135 mmHg, who was a non-smoker, with no CVD family history.
When risk scores were calculated, Patient A (without psoriasis) had a 22% risk of having a cardiovascular event in the next 10 years compared with Patient B (with psoriasis), who had a 7% risk. The risk for Patient B was initially calculated as 4.6%, explained Prof Sattar, but became 7% when multiplied by 1.5 to take psoriasis into consideration (which in this case was severe and of early onset). These case studies highlight that just because a patient has early-onset psoriasis and a high BMI, they are not automatically at a higher risk of CVD. It is important, he said, to take all risk factors into consideration, since many other specific factors can place patients at high risk, irrespective of the nature of their psoriasis.
The best evidence for lowering CVD risk, said Prof Sattar, comes from statin trials. The Joint Societies risk calculator shows statins lower CVD risk by 20–25% per mmol/L reduction in low density lipoprotein-cholesterol.30 The risk calculator can be used to demonstrate how much longer patients can expect to live if they take preventive actions. Statins should be considered if patients are above the risk threshold, and blood pressure should be treated according to national guidelines. Helping patients stop smoking, Prof Sattar added, provides huge benefits with a wide variety of techniques available (patches, tablets, and cognitive behaviour therapy).
Turning to diabetes and metabolic risks, Prof Sattar said that evidence suggests that psoriasis and obesity are interlinked; of people with a BMI >27, 28% have PsA versus 15% with RA. There is evidence that obesity is 47% more common in patients with severe versus mild psoriasis, and that response of patients to systemic therapy is inversely proportional to BMI.
Prof Sattar presented the case of a 38-year-old male non-smoker who had 5 years of mild psoriasis with no joint disease. Blood pressure was 138/78, weight 88 kg, height 1.7 m, and BMI 31.2. Test results showed haemoglobin A1c was 43 mmol/mol, alanine transaminase 53, aspartate transaminase 32, cholesterol 4.9 mmol/L, high-density lipoprotein-c 1.1, and triglycerides 3.5 mmol/L.
The patient has a higher risk of developing diabetes than CVD since haemoglobin A1c was at a prediabetic level, and he has evidence of NAFLD. His CVD risk, however, was lower due to his youth and being a non-smoker with normal blood pressure. If psoriasis and PsA patients are overweight or obese, said Prof Sattar, they should undergo cholesterol (including triglycerides and high-density lipoprotein cholesterol) and liver function testing.
Weight reduction, said Prof Sattar, delivers a variety of benefits, including improved quality of life, direct or indirect improvement of psoriasis, and reduced risk of diabetes, NAFLD, and CVD.26 New National Institute for Health and Care Excellence (NICE) guidance suggests a 3% weight loss is realistic.31 It was far easier to lose weight through dietary changes than physical exercise. The focus, he added, should continue to be on smoking cessation and sensible alcohol intake. Remember, Prof Sattar concluded, to be kind to patients and not to give them a hard time, because weight loss can be difficult to sustain; doctors should also remember most patients do not wish to be overweight or obese, so they deserve our empathy and help.
In the question and answer session Prof Sattar was asked if treatment of psoriasis reduces CV risk. Prof Sattar said the CANTOS trial provided proof of concept that damping systemic inflammation reduces CV risk. Additional support comes from observational data on anti-TNF. Other risk factors, such as smoking, will need to be taken into consideration.
Understanding the Rheumatic Comorbidities of Psoriasis
Doctor Frank Behrens
Data suggest, said Dr Behrens, that 70% of patients with pre-diagnosed psoriasis go on to develop PsA, 15% have PsA prior to skin disease, and 15% have concomitant onset of PsA and skin disease. So how do clinicians decide whether an individual patient with psoriasis is at risk of developing PsA?30
A cross-sectional observational study demonstrated that patients who have had psoriasis for 27 years have the same probability of developing PsA in one year as those who have had psoriasis for 1 year (Figure 3).32 For clinicians, said Dr Behrens, the bottom line is you have to ask about joint complaints at every consultation, because patients have the same risks of PsA regardless of whether it is their first or twenty-first visit.
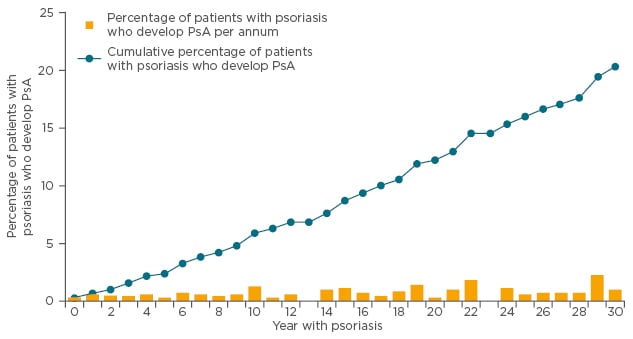
Figure 3: Prevalence of PsA in patients with psoriasis: Incidence and cumulative prevalence of PsA among patients with psoriasis treated by dermatologists.
PsA: psoriatic arthritis.
Adapted from Christophers et al.32
Such data suggest that psoriasis and PsA are the same disease, with PsA occurring later in the disease course. A paired genetic analysis of synovial and skin tissue revealed that gene expression profiles for PsA synovial joints were more tightly linked to skin psoriasis than RA.33 Such links, said Dr Behrens, indicate it may be better to select drugs for PsA that work in psoriasis, opposed to RA.
Currently there are two alternative hypotheses for PsA genetic predisposition. The first is that all risk alleles for skin diseases also cover the total heritability of PsA peripheral joint manifestations and PsA axial manifestations. The second is that risk alleles for skin disease and different phenotypes of PsA only partially overlap.34
With its limited genetic pool, Iceland provides valuable insights into risks of family members developing different diseases. In one study, national identification numbers of 220 Icelanders known to have PsA were linked with genealogy databases and risk ratios were estimated for first to fifth-degree relatives matched to unaffected controls from the Icelandic population.35 Results showed RR for developing PsA from first to fourth-generation were 39.0, 12.0, 3.6, and 2.3, respectively (all p<0.0001); whereas the fifth-degree relatives had an RR of 1.2 (p=0.236). These results therefore indicate a strong genetic component.
If psoriasis and PsA were more or less the same disease, said Dr Behrens, similar numbers of patients would be found in second, third, and fourth generations, which was not the case. First-degree relatives of patients with psoriasis have between 4 to 10-fold increased risk of developing the skin condition, far lower than the 39-fold increase in first-degree relatives of PsA patients. PsA, it appears, has a higher genetic component than psoriasis.35
In a genome study, significant associations with the PTPN22 loci were found for PsA susceptibility, but not for psoriasis.36 Such data, said Dr Behrens, suggest PsA and psoriasis are different diseases but are linked. A model where mice spontaneously develop arthritis after 6–12 months (due to STAT 3C overexpression) showed that when psoriasis is induced experimentally, mice immediately develop PsA.37 Psoriasis appears to act as a trigger for developing PsA in those with a genetic predisposition.
With such links between psoriasis and PsA, dermatologists are in a good position to identify PsA early. In a logistic regression analysis of patients with psoriasis, the strongest predictors for concomitant PsA were nail involvement (odds ratio: 2.93) and inpatient hospital treatment (odds ratio: 1.63).38 The current recommendation for screening patients with psoriasis for PsA is once a year using screening questionnaires.
To meet Classification Criteria for Psoriatic Arthritis (CASPAR), patients must have inflammatory articular disease (joint, spine, or entheses) with ≥3 points from five additional categories.39 Dr Behrens cautioned that it can take 6 months to 1 year for swollen joints, osteoproliferation, and erosions to develop, and that they were not seen in every patient. Patients may be free from joint swelling, but found to have periostitis and bone marrow oedema on magnetic resonance imaging (MRI). Other patients who are negative on clinical examination may show slight inflammation with fluorescent optimal imaging or ultrasound.
According to the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment recommendations for patients with peripheral arthritis, axial disease, enthesitis, and dactylitis, conventional synthetic disease-modifying antirheumatic drugs, such as methotrexate, should be used in peripheral arthritis and dactylitis, but not in axial disease and enthesitis.40
There is a need, said Dr Behrens, to individualise PsA treatment to phenotype. For patients with skin, dactylitis, and enthesitis manifestations, and arthritis, there is evidence of efficacy with leflunomide, apremilast, methotrexate, cyclosporine, and sulfasalazine. However, for patients with skin, enthesitis, and dactylitis manifestations, but not arthritis, evidence suggests going directly to biologics (Behrens F. Personal communication).
In rheumatology, only around 30% of patients achieve an American College of Rheumatology score of 50% symptom improvement (ACR 50), making it important to optimise outcomes. A study by Behrens on optimisation of anti-TNF monotherapy showed 43% of patients achieved good quality of life scores, 56.6% achieved good skin scores, and 72.5% good arthritis scores; however, only 24.3% achieved simultaneously good responses in all three domains. The addition of conventional synthetic disease-modifying antirheumatic drugs increased the percentage of patients achieving good skin scores to 75.5%; although, this was at the expense of arthritis scores and made no difference to the achievement of good scores in all three domains (Behrens F. Data on file).
The challenge for the future, Dr Behrens concluded, is to optimise outcomes by finding the right treatments for individual patients.
The Link Between the Skin and the Gut: Inflammatory Bowel Disease in Patients with Psoriasis
Doctor Krisztina Gecse
IBD, such as UC and CD, said Dr Gecse, are defined as a chronic inflammation in the gastrointestinal (GI) tract induced by “inappropriate and continuing inflammatory response to commensal microbes in a genetically predisposed individual.”41
IBD aetiology includes genetic susceptibility, environmental triggers, luminal microbial antigens and adjuvants, and aberrant immune responses.41 The natural course of CD shows most patients start with inflammatory disease and later experience stricturing or penetrating complications (abscesses and fistulas).42 However, a significant proportion of CD patients already present with penetrating or stricture disease. With UC, approximately 20% of patients will have undergone colectomy after 20 years of disease duration.43
The disease course of IBD follows four principal scenarios, of which the most common are remission or mild severity of intestinal symptoms after initial high activity (affecting 43% of CD patients and 59% of UC patients) and chronic intermittent symptoms (affecting 32% of CD patients and 31% of UC patients).43
A number of prognostic factors have been associated with complicated CD including young age at disease onset, smoking, severe upper GI disease, extensive small bowel disease, perianal disease, need for steroids at diagnosis, weight loss, and deep ulcerations upon index endoscopy.
CD can affect any segment of the GI tract, most commonly the terminal ileum, as indicated by its Latin name, ileitis terminalis.44 UC, in contrast, is a diffuse, continuous disease which typically starts in the rectum and spreads upwards, with no ileal involvement (except backwash ileitis) and is not associated with perianal lesions.45 Signs and symptoms for CD include abdominal pain, weight loss, chronic diarrhoea, malaise, anorexia, and fever;44 while signs and symptoms for UC most commonly include bloody diarrhoea, rectal bleeding, tenesmus, abdominal pain, urgency, faecal incontinence, nocturnal defecation, fatigue, anorexia, and weight loss.45 Clues to IBD diagnosis include chronic symptoms, systemic symptoms, young age, and family history of IBD. However, there is no single gold standard method for diagnosing IBD, therefore a combination of clinical evaluation, biomarkers, endoscopy, histology, and imaging should be taken into account.44,45
The link between the skin and the gut can be approached from three directions; psoriasis and IBD as true comorbidities, psoriasis as paradoxical reaction to anti-TNF medication, and IL-17 inhibitors in the treatment of psoriasis. IBD patients, said Dr Gecse, are routinely screened for extra-intestinal manifestations including psoriasis. Prevalence rates of CD and UC are approximately four-times higher in the psoriasis population than in the general population, with the highest risks occurring in patients with both psoriasis and PsA. Psoriasis is more strongly associated with CD than UC, with Psoriasis-CD patients having milder psoriasis but earlier onset and more severe Crohn’s phenotypes.46
Since the IL-17 pathway in the pathogenesis of IBD and psoriasis are shared, it seemed plausible to investigate IL-17 inhibitors for the treatment of IBD as well. However, both anti-IL-17A monoclonal antibodies secukinumab and brodalumab induced worsening of CD in moderate-to-severe CD, and showed the area under the curve analysis significantly favoured placebo (p=0.043).47 A second Phase II study, this time using brodalumab in moderate-to-severe CD, showed the proportion of patients with worsening disease was lowest for placebo and the Crohn’s Disease Activity Index (CDAI) higher in brodalumab.48
IBD patients undergoing anti-TNF treatment can show paradoxical skin reactions. The prevalence of new-onset psoriasis with anti-TNF is known to be 0.6–5.3%,49,50 with the most frequent presentation being palmoplantar.49-51 Skin lesions can appear at any time after initiation of anti-TNF treatment, most frequently after the third infliximab infusion.51-53 In most cases, topical treatment is sufficient; however, stopping anti-TNF and initiating other systemic treatments may be necessary.49
IBD patients represent a broad disease spectrum in terms of disease location, severity, complications, and comorbidities. In an ideal world, biopsy samples, serum samples, or faecal stool tests would allow patient profiling and personalised medicine, determining the most optimal choice of treatment to reach mucosal healing, to avoid complications and clinical symptoms, and ultimately leading to improved quality of life.
In the question and answer session, when asked about symptoms and laboratory results that should alert rheumatologists to refer patients for IBD assessments, Dr Gecse said young patients with a family history of IBD, chronic abdominal, and possibly systemic symptoms should be referred to a gastroenterologist. Additionally, Dr Gecse added, faecal calprotectin is a good biomarker to distinguish between functional GI disorders and IBD.
Click here to view the full symposium.







