Presenter: Alexa Kimball1
Presenters: Afsaneh Alavi,2 Gregor Jemec,3,4 Alice Gottlieb,5 Xiaoling Wei,6 Magdalena Wozniak,7 Lorenz Uhlmann,8 Angela Llobet Martinez,8 Deborah Keefe,9 Ruvie Martin,9 Li Chen,8 Elisa Muscianisi9
1. Harvard Medical School and Clinical Laboratory for Epidemiology and Applied Research in Skin (CLEARS), Department of Dermatology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA
2. Department of Dermatology, Mayo Clinic, Rochester, Minnesota, USA
3. Department of Dermatology, Zeeland University Hospital, Roskilde, Denmark
4. Health Sciences Faculty, University of Copenhagen, Denmark
5. Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York, USA
6. Novartis Pharma Shanghai, China
7. Novartis Ireland Limited, Dublin, Ireland
8. Novartis Pharma AG, Basel, Switzerland
9. Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA
Disclosure: Kimball is a consultant and investigator for Abbvie, Bristol Meyers Squibb, Janssen, Eli Lilly, Novartis, Pfizer, and UCB; an investigator for Incyte and AnaptysBio; a consultant for Bayer, Boehringer Ingelheim, Ventyx, Moonlake, Eli Lilly, Concert, EvoImmune, Sonoma Bio, and Sanofi; receives fellowship funding from Janssen; and serves on the Board of Directors for Almirall. Alavi has received honoraria as a consultant or advisory board participant from AbbVie, Janssen, Novartis, Boehringer Ingelheim, InflaRx, and UCB; and is an investigator for Processa and Boehringer Ingelheim. Jemec has served as a consultant for AbbVie, Coloplast, Leo Pharma, Novartis, UCB, and InflaRX; as an investigator for AbbVie, Leo Pharma, Novartis, Regeneron, UCB, and InflaRX; has received unrestricted grants from AbbVie, LeoPharma, and Novartis; has served on advisory boards for AbbVie, Janssen Pharmaceuticals, MSD, and Novartis; and as a speaker for AbbVie, Coloplast, Leo Pharma, and Galderma. Gottlieb has received honoraria as an advisory board member, non-promotional speaker, or consultant for Amgen, Anaptsys Bio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi, Sun Pharmaceutical Industries, UCB, and Xbiotech; and research/educational grants from AnaptysBio, Janssen, Novartis, Ortho Dermatologics, Sun Pharmaceutical Industries, Bristol Myers Squibb, and UCB. Wei is an employee at Novartis Pharma Shanghai, China. Wozniac is an employee and stockholder at Novartis Ireland Limited, Dublin. Uhlmann and Martinez are employees and stockholders at Novartis Pharma AG, Basel, Switzerland. Keefe, Martin, Chen, and Muscianisi are employees and stockholders at Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA.
Acknowledgements: Writing assistance was provided by Helen Boreham, Wetherby, UK.
Support: The publication of this article was funded by Novartis Pharma AG, Basel, Switzerland.
Citation: EMJ Dermatol. 2022;10[1]:40-43. DOI/10.33590/emjdermatol/10182311. https://doi.org/10.33590/emjdermatol/10182311.
Meeting Summary
The primary endpoint analyses from the SUNSHINE and SUNRISE Phase III trials of secukinumab in moderate-to-severe hidradenitis suppurativa (HS) were featured in an oral presentation as part of the late-breaking news session at the 31st annual European Academy of Dermatology and Venereology (EADV) congress. Known collectively as the SUNNY trials, the SUNSHINE and SUNRISE studies represent the largest Phase III trials conducted in HS to date. The primary endpoint was met in both studies for the every 2 weeks (Q2W) regimen, and in one for the every 4 weeks (Q4W) regimen, demonstrating the superiority of secukinumab over placebo in patients with moderate-to-severe HS. Secukinumab delivered rapid symptom relief and showed a favourable safety profile consistent with its use in other indications. Available data on file from the Week 52 database lock support the sustained efficacy beyond Week 16 with no new safety findings. Based on the positive outcomes of these milestone clinical trials, secukinumab is expected to be a new, safe, and effective biologic treatment option with a novel mode of action, targeting IL-17A, for moderate-to-severe HS.Primary Endpoint Analysis from the SUNSHINE and SUNRISE Phase III Trials
Alexa Kimball, Harvard Medical School and Clinical Laboratory for Epidemiology and Applied Research in Skin (CLEARS), Department of Dermatology, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, presented the Week 16 results from the two identical Phase III SUNSHINE and SUNRISE trials.1 Both studies were randomised, double-blind, multicentre clinical trials that assessed the short- (16 weeks) and long-term (up to 1 year) efficacy, safety, and tolerability of secukinumab, a monoclonal antibody selectively neutralising IL-17A in adult patients with moderate-to-severe HS. Patients were randomised 1:1:1 to one of two subcutaneous secukinumab dosing regimens: 300 mg Q2W or 300 mg Q4W, or placebo. Key inclusion criteria included ≥5 inflammatory lesions affecting at least two distinct anatomical areas at baseline, and diagnosis of HS ≥1 year prior to enrolment. Patients in the antibiotic stratum were permitted to enter the study on stable treatment with selected antibiotics (i.e., doxycycline, minocycline, and tetracycline, representing only 10–14% of patients). Patients with HS with a total fistulae count ≥20 at baseline, active inflammatory disease, or previous exposure to secukinumab or other IL-17(A)-biologics were excluded. Together, the SUNSHINE and SUNRISE trials randomised a total of 1,084 patients with HS across 219 sites worldwide between January 2019 and July 2022. Retention was very high, with a completion rate of approximately 94% across the two studies. Overall, patients were well-balanced between treatment arms, although Kimball highlighted the higher proportion of Hurley Stage III patients in the SUNRISE trial, particularly in the secukinumab Q2W arm (45.6%), indicating a more severe patient population.
Both the SUNSHINE and SUNRISE studies met their primary endpoint based on HS clinical response (HiSCR), defined as at least a 50% decrease in abscess and inflammatory nodule (AN) count with no increase in the number of abscesses, or in the number of draining fistulae relative to baseline (Figure 1). Across both studies, numerically greater HiSCR response rates for secukinumab compared to placebo were seen at all time points from Week 2 to Week 16, with secukinumab demonstrating a rapid onset of action by Week 2. In the SUNSHINE and SUNRISE trials, treatment with secukinumab 300 mg Q2W demonstrated superiority compared to placebo in regard to the proportion of HiSCR responders (primary endpoint) in patients with moderate-to-severe HS at Week 16. In the SUNRISE trial only, secukinumab 300 mg Q4W was superior versus placebo at Week 16 for HiSCR. Despite not achieving superiority, numerical differences at Week 16 were also observed in the SUNSHINE trial following the Q4W dosing regimen. The clinical response (HiSCR) to secukinumab at Week 16 is in line with the sustained and continued improvement that was seen up to 52 weeks of treatment, based on available data on file from the Week 52 database lock.
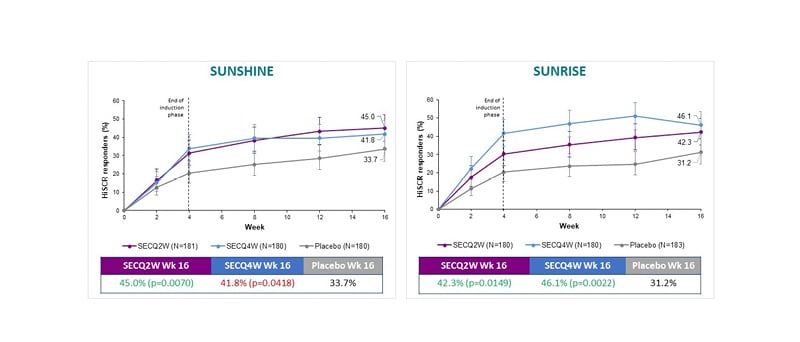
Figure 1: SUNSHINE and SUNRISE both met their primary endpoints.
HiSCR: hidradenitis suppurativa clinical response; Q2W: every 2 weeks; Q4W: every 4 weeks; SEC: secukinumab; Wk: Week.
Secukinumab also achieved statistical significance and clinical improvements in key secondary endpoints evaluated in the SUNSHINE and SUNRISE trials. In both studies, secukinumab significantly reduced the AN count in patients with moderate-to-severe HS. The decrease in AN count with secukinumab appeared as early as Week 2 of treatment, and further improved up to Week 16. Overall, patients in the secukinumab treatment groups attained reductions from baseline of 39–47% in their AN count by Week 16, compared to decreases of approximately 23% with placebo.
Secukinumab was also shown to reduce the number of flares experienced by patients with moderate-to-severe HS, an outcome that Kimball described as an important “mirror” of efficacy. Across both studies, the proportion of patients experiencing flares was lower with secukinumab versus placebo at all time points from Week 2 to Week 16. Again, secukinumab demonstrated a rapid onset of action, with improvement seen as early as Week 2.
Finally, secukinumab reduced pain in patients with moderate-to-severe HS, which Kimball highlighted as an important and debilitating feature of this disease. A significantly higher proportion of patients with HS achieved a skin pain response (using the high hurdle of the numeric rating scale [NRS30] score), analysed based on pooled data from SUNSHINE and SUNRISE, compared with placebo at Week 16 following secukinumab 300 mg Q2W treatment. Despite not achieving statistical significance, numerical superiority at Week 16 was also observed with the Q4W dosing regimen. Only patients with a baseline NRS ≥3 were included in this analysis of skin pain.
Alongside its clinical efficacy, secukinumab also demonstrated substantial improvements in the quality of life of patients with moderate-to-severe HS in exploratory analyses from the SUNSHINE and SUNRISE trials. In both studies, Dermatology Life Quality Index (DLQI) response, defined as a >5 point reduction, was evident as early as Week 2. The proportion of patients achieving DLQI response was greater in both secukinumab regimens at Week 16 compared to placebo.
Safety data from the SUNSHINE and SUNRISE trials showed secukinumab to be well tolerated, consistent with its established safety profile in other disease states. Rates of adverse events (AE) and all non-fatal serious AEs were very similar in both secukinumab arms compared to placebo. Rates of infections, including Candida infections, were low and not substantially different to placebo. The number of patients discontinuing study treatment due to AEs was also low across the secukinumab treatment groups in both trials. Discontinuations due to AEs in the secukinumab Q2W and Q4W arms were 2.8% and 0.6%, respectively, in the SUNSHINE trial and 0.6% and 2.2%, respectively, in the SUNRISE trial, compared to discontinuation rates of 0.6% and 2.2%, respectively, for placebo. Kimball described these safety data as “reassuring”, with secukinumab demonstrating “a very clean profile overall.”
Conclusion
Both the SUNSHINE and SUNRISE Phase III trials successfully met the primary endpoint, demonstrating superiority of secukinumab over placebo in HiSCR, with rapid symptom relief in patients with moderate-to-severe HS. Secukinumab reportedly also demonstrated sustained efficacy beyond the Week 16 primary efficacy analysis. Overall, secukinumab was well tolerated in patients with moderate-to-severe HS, consistent with its known safety profile. Collectively, these results highlight that secukinumab is expected to be a new, safe, and effective addition to the treatment armamentarium for moderate-to-severe HS, Kimball concluded.







