Meeting Summary
The main objectives of the symposium were to define the need for ambitious goals in the treatment of psoriasis, including the importance of clear skin, from the patients’ perspective and to discuss the considerations that make a difference in ensuring that the right treatment reaches the right patient. The final aim was to consider what else dermatologists can be doing to help patients beyond the prescribed medication. Prof Kirby started the symposium by introducing a thought-provoking video of a patient describing his psoriasis journey and the challenges he faced in finding the right treatment. Prof Kirby went on to encourage the panel and audience to provide their views on the critical questions that tell us how we should be managing patients with moderate-to-severe psoriasis. Prof Mrowietz then involved the audience in a discussion of which drug features they consider to be the most important when finding the optimum treatment for patients, particularly now that more treatment options are available. Prof Mrowietz highlighted the importance of a patient-centric approach in treatment selection and the impact that psoriasis has beyond the skin. Prof Iversen presented on the need for ambitious treatment goals in moderate-to-severe psoriasis, beyond Psoriasis Area Severity Index (PASI) 90 and towards absolute PASI values, and presented the evidence linking higher PASI goals with improved quality of life. Prof Iversen concluded the symposium with a discussion on the management of comorbidity and risk factors in moderate-to-severe psoriasis.
What is the Patient Perspective on Treatment Goals in Psoriasis?
Professor Brian Kirby
Dermatologists frequently see psoriasis patients for whom the most troublesome symptoms are not the constant physical discomfort, but rather the feelings of anxiety and depression that so often accompany the disease.1 Even when patients find a treatment that works for them, they are still anxious that the treatment may stop working and their symptoms could return. These patients are a reminder of the need to focus not only on the skin, but to consider the wider implications of psoriasis and the impact that good treatment can have on the whole person.
From both the patients’ and physicians’ perspective, there are many factors involved in deciding on the right treatment. Physicians are primarily looking for treatments that provide sustained efficacy on the skin, a good safety profile, and a fast onset of action, whilst many patients want to find a treatment that will completely remove the emotional burden of psoriasis and the fear of its return. The availability of biologic systemic treatments makes clear skin an achievable target for most people.2 These newer treatments provide the needed sustained control of >12 months for what is a lifelong disease for most patients who develop psoriasis in their teens or early twenties. This level of long-term efficacy goes beyond the short-term efficacy that physicians are familiar with from established treatments such as cyclosporine, which provides good results in the short term but is not now preferred as a long-term option. Unfortunately, the higher cost of biologic agents means that treatment choices are, to some extent, guided by healthcare system constraints.
In summary, while there are many factors to consider in selecting the right treatment, it is important that physicians understand what is most important to their patients to find the right treatment for them quickly, therefore avoiding unnecessary treatment costs and the prolonged psychological harm of living with uncontrolled psoriasis.
Psoriasis Complex: What is an Optimal Treatment?
Professor Ulrich Mrowietz
Physicians should have a patient-centric approach to psoriasis treatment, understanding the disease from the patients’ perspective to find the treatment that will have the best results. To do this, it is important to consider that psoriasis is not just a skin condition but a complex combination of genetic predisposition, risk factors, triggers, and associated serious comorbidity, including cardiovascular and psychiatric diseases, and that modifying or managing risk factors and comorbidity may improve patient outcomes (Figure 1).3,4 The most startling evidence of the impact of psoriasis beyond the skin is that people with severe psoriasis have a higher mortality risk than the general population,5 including increased rates of major cardiovascular events, such as myocardial infarction and stroke.6,7 The relationship between psoriasis, atherosclerosis, and cardiovascular risk may be due to a shared pathology involving key immunological cell populations and common inflammatory mediators, including the IL-17 family of cytokines.8 Studies are ongoing to assess if biologic agents targeting IL-17 are able to modify vascular inflammation in patients with psoriasis.
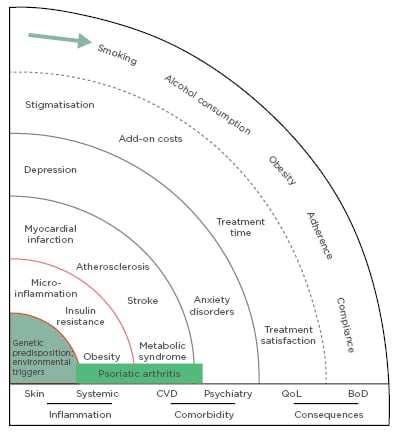
Figure 1: The spheres of psoriasis disease.
BoD: burden of disease; CVD: cardiovascular disease; QoL: quality of life.
Adapted with permission from Mrowietz et al.4
Right Treatment to the Right Patient
As more treatment options have become available, expectations of what makes a good treatment have also shifted. When patients ask what they can expect from a treatment, physicians want to be able to tell them that the treatment will give them clearer skin for as long as they are taking it and that it will be safe and tolerable for long-term use. Therefore, it is important that clinicians have the right tools to select the most appropriate treatment for each patient.
Most dermatologists have the traditional systemic agents available to them: methotrexate, acitretin, cyclosporine, and dimethyl fumarate. These drugs are well established and supported by treatment guidelines,9 and as low-cost options, they are often required by our local health systems to be used as first-line treatments. Conventional systemic therapies, although a lower-cost option, are often limited in their ability to provide the long-term improvements that patients value and may not work for a proportion of patients. For example, in a study of an intensified dosing schedule of subcutaneous methotrexate, the proportion of patients achieving a 75% reduction in PASI score (PASI 75) at Week 16 was 41%.10 This is in contrast to the results achieved in the AMAGINE-2 and AMAGINE-3 Phase III studies of the anti-IL-17 receptor (R) monoclonal antibody brodalumab, in which PASI 75 was achieved by 86% and 85% of patients receiving brodalumab 210 mg at 12 weeks, respectively.11 There are now more biologic agents available than conventional systemic treatments, with adalimumab biosimilars available in some areas, adding to the list of options.
With a wealth of options available, what factors should physicians consider when finding the right treatment for their patients? Most treatment guidelines have not been updated since the biologics targeting IL-17 and IL-23 have been approved; therefore, there is limited guidance on the order in which all the available treatments should be used. Existing guidelines cover the earlier biologics, such as TNF inhibitors adalimumab, etanercept, and infliximab, positioned as second line to methotrexate, cyclosporine, and acitretin.12 Although most of these biologics are approved for first-line use, most healthcare systems and insurers require patients to be treated with less expensive, traditional agents before escalating to more expensive biologics,13 despite evidence suggesting superior efficacy of newer biologics. For example, a recent network meta-analysis assessed the long-term outcomes with novel biologic and nonbiologic systemic therapies from 24 studies in patients with moderate-to-severe psoriasis, with outcomes measured at or around 1 year. The analysis found that brodalumab was associated with a higher likelihood of sustained PASI response compared to secukinumab, ustekinumab, etanercept, adalimumab, apremilast, infliximab, and ixekizumab (Figure 2).14
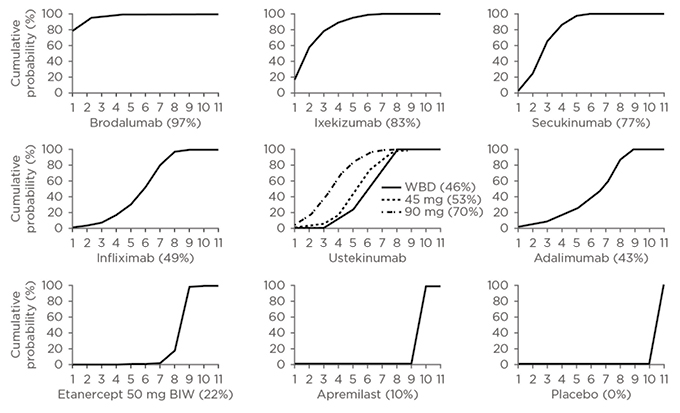
Figure 2: Cumulative ranking of novel systemic therapies for moderate-to-severe psoriasis.
Cumulative ranking probability plots and SUCRA for each treatment included in network meta-analysis of 52-week randomised, controlled trials using induction-phase placebo control. On the horizontal axis is the possible rank of each treatment according to the magnitude of its treatment effect across all measures of PASI response (from the best [1] to the worst [11] ranks). On the vertical axis is the cumulative probability for each treatment to be the best option, among the best two options, among the best three options, and continuing. If a treatment always ranks first, then the SUCRA=100%; if a treatment always ranks last, then the SUCRA=0%.
BIW: twice a week; SUCRA: surface under the cumulative ranking curve; WBD: weight-based dosing.
Reproduced from Sawyer et al.14 under the terms of the Creative Commons Attribution Non-Commercial No Derivatives License CC BY-NC-ND.
Guidelines for the most appropriate long-term options should therefore be updated once evidence from more long-term comparative studies, and particularly real-world studies, of newer treatments is available.
Following efficacy and safety, patient convenience is an important consideration, and patients’ preference for an oral conventional systemic or an injectable biologic could make a big difference in their adherence to treatment. Adherence to biologic therapy is still unacceptably low. A recent study estimated biologic nonadherence to be roughly 25%,15 which is an improvement on nonbiologic and topical treatments, but is still too low, especially considering the high cost of these drugs.
In summary, now that there are many efficacious treatments available for moderate-to-severe psoriasis, it is more important than ever for physicians to understand which features of a treatment are most aligned with their patients’ needs.
Treatment Expectations
It is important that physicians consider if their expectations for treatment outcomes are aligned with their patients’ expectations. In a recent analysis of the two large German and Swiss patient registries PsoBEST and SDNTT,16 in which 5,343 patients with psoriasis were studied between 2008 and 2016, the most often reported needs were to ‘get better skin quickly’ and ‘to be healed of all skin defects’. Women and those <65 years of age had higher expectations of their treatment.16 Until relatively recently, there was no consensus on how to translate these expectations into clinical measures. Treatment goals define treatment failure and indicate when physicians need to change treatment and have been used for decades in other diseases, e.g., HbA1c in diabetes and blood pressure targets for hypertension. In 2011, however, two goals for moderate-to-severe psoriasis were defined: a definition for treatment failure equating to a <50% reduction in PASI (PASI 50) and a definition of treatment expectation of a reduction in PASI of 75% or more (PASI 75).17
Using a relative value for PASI as a treatment goal may still not provide the detail required to effectively measure response. Indeed, even achieving relative PASI 75 from baseline, absolute PASI 40 will still only achieve absolute PASI 10, which is the threshold for moderate-to-severe psoriasis. Relative PASI values are a good measure of response, particularly in clinical trials in which there is often a true baseline due to patients undergoing a washout phase; however, in real life, relative PASI may be affected by prior treatment and absolute PASI could be more relevant. In a move towards alignment between relative and absolute PASI, relative and absolute PASI were compared in a recent study of ixekizumab and found that relative PASI 90 corresponded very well to absolute PASI ≤2 and, similarly, relative PASI 75 corresponded well with absolute PASI ≤5.18
As more highly efficacious biologic therapies become established as long-term maintenance therapy, complete clearance and absolute PASI values are becoming realistic treatment targets that align with the high expectations patients have for their treatment.
Patient-Reported Outcomes
Patient-reported outcomes are a valuable tool for assessing patients and for selecting a treatment, whether they take the form of conversations to ascertain patients’ expectations of treatment, or formal assessments using the Dermatology Life Quality Index (DLQI). The authors’ existing consensus on treatment goals17 incorporated DLQI to differentiate patients whose relative treatment outcome was between PASI 50 and 75. If the patient’s perspective was adequate with DLQI of ≤5, then it was considered reasonable to continue treatment, even at PASI <75. DLQI should not, however, be used instead of full conversations with patients to fully appreciate their perspective on the severity of their symptoms.
The presence of particular symptoms can contribute to the perceived severity of psoriasis and therefore influence treatment choice and escalation. Studies have highlighted differences in patient and physician perspectives on the most debilitating symptoms; in particular, it was reported that patients consider itch to be the most important factor contributing to disease severity,19 whereas physicians consider the location and size of lesions to be more important.20
This, once again, highlights the very real need for robust assessment tools for patient-reported outcomes and thorough discussion between patients and prescribers about symptoms and treatment expectations.
How Have Treatment Expectations Changed in the Era of New Biologic Agents?
Professor Lars Iversen
As an increasing number of new agents become available, many physicians are more likely to intensify treatment more quickly, when in the past they would have adjusted the dose or dose frequency, or added another treatment, rather than switch to a new therapy. This change in behaviour goes hand in hand with the movement towards better treatment goals for moderate-to-severe psoriasis.
Since the European consensus on treatment goals was published in 2011,17 the recent availability of new agents suggests these recommendations for response assessment and modification strategy need to be updated. Only this year, the French Society of Dermatology published new guidelines on the use of systemic treatments for adult patients with moderate-to-severe psoriasis.21 Whereas the old consensus used a lower threshold of relative PASI 50 for treatment modification, new French guidelines have raised the bar to relative PASI 75 or absolute PASI >3 as the cut-off for requiring treatment modification. As reflected in current practice, the cut-off at which it is reasonable to continue with the selected treatment regimen has also been raised and adjusted to include assessment of absolute PASI and is now relative PASI ≥90 and/or absolute PASI <3 (Figure 3).21 Evaluation of patient satisfaction is advocated throughout the guidelines, specifically through the use of DLQI for patients who fall between relative PASI ≥75 and <90, and assessment for involvement of key sites (nails, face, scalp, palms, soles, genitals) is specified to be included in the decision to modify or continue treatment.
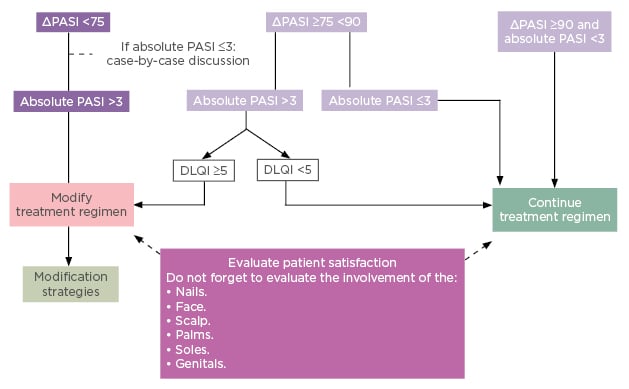
Figure 3: French treatment goals 2019.
DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index.
Reproduced from Amatore F et al.21 under the terms of the Creative Commons Attribution Non-Commercial No Derivatives License CC BY-NC-ND.
Although these guidelines are only from one country, they are a good indicator of the future direction of treatment goals, capturing both DLQI and absolute PASI values. An analysis of the AMAGINE-1, -2, and -3 studies on a combined pool of 4,373 patients looked at the correlation between the proportion of patients achieving DLQI 0 and PASI response.22 The study demonstrated a strong positive correlation between DLQI and PASI response, with 67% of PASI 100 responders and 40% of PASI ≥90 to <100 responders achieving DLQI 0,22 supporting the inclusion of these parameters in treatment targets.
New Biologic Agents Are the Difference
The availability of new biologic agents for treatment of moderate-to-severe psoriasis is the driving force behind the need for improved treatment goals, but what is it about these agents that makes the difference? There is now a long list of agents targeting a number of cytokines involved in psoriasis pathogenesis, including TNF, IL-23, IL-17A, the wider IL-17 family, and the IL-17RA subunit. The pathology of psoriasis is complex, with the involvement of many cell types, and there are multiple signalling pathways at play in what can be described as a complex cycle with multiple points for potential intervention.23
Agents Targeting IL-23
Three of the recently approved biologic agents, guselkumab, tildrakizumab, and ustekinumab, are monoclonal antibodies targeting IL-23.
IL-23 is a heterodimeric cytokine consisting of two subunits: p19, which is unique to IL-23, and p40, which is shared with IL-12.24 The role of IL-23 in the pathogenesis of psoriasis is to activate and maintain the T-helper 17 (Th17) pathway, stimulating Th17 and other immune cells to produce and express another inflammatory cytokine, IL-17A.25 Guselkumab and tildrakizumab target the IL-23p19 subunit, and ustekinumab targets the IL-12/23p40 subunit.
Agents Targeting the IL-17 Cytokine Family
The IL-17 family consists of six cytokine mediators of acute and chronic inflammatory response (IL-17A to IL-17F).26 IL-17 is produced by CD4+ Th17 cells and CD8+ cytotoxic T cells of the adaptive immune system and also by cells of the innate immune system: γδ-T cells, macrophages, and mast cells.27 All of the IL-17 family signals through heterodimeric complexes of the IL-17R family.28 IL-17A is known to be a major driver of inflammatory autoimmune diseases, including psoriasis29,30 and rheumatoid arthritis (RA),31 through regulating expression of a wide array of pro-inflammatory mediators in tissue cells, including keratinocytes.32-34 Increased levels of IL-17A are present in lesional and nonlesional skin30,35 and in the serum36 of patients with psoriasis compared with healthy patients, and it is therefore an important target for therapy of psoriasis. Two of the approved biologics, secukinumab and ixekizumab, selectively target IL-17A and suppress expression of the specific inflammatory genes it regulates.
Brodalumab is the only approved agent targeting the IL-17RA subunit of IL-17R,37 through which IL-17A and three other members of the IL-17 family (IL-17C, IL-17E, and IL-17F) all signal.38 IL-17C, IL-17E, and IL-17F have been shown to have varying degrees of involvement in psoriasis pathogenesis in addition to IL-17A.39 IL-17F and IL-17C share 55% and 23% sequence homology with IL-17A, respectively,40,41 and both are overexpressed in lesional psoriatic skin compared with nonlesional and normal skin.40,42 The expression of IL-17E (also known as IL-25) in psoriatic skin is less well defined.42,43 However, a recent study showed that IL-17E blockade greatly impaired keratinocyte proliferation and epidermal hyperplasia in a psoriasis-like mouse model.44 As the pathogenesis of psoriasis is so complex, it is very difficult to assess if one cytokine pathway is more important than another as a treatment target. However, there is evidence to suggest that targeting the IL-17RA subunit may be more effective than blocking the individual cytokines. For example, brodalumab was ranked as having a higher treatment effect in terms of sustained PASI response compared with secukinumab and ixekizumab, among other agents.15 Likewise, the Phase III AMAGINE-2 and AMAGINE-3 studies showed that brodalumab at a dose of 210 mg every 2 weeks provided a significantly higher PASI 100 response rate at Week 12 compared with ustekinumab.11
Further comparative studies are ongoing, with the potential to determine if any treatments are more effective for particular patient subgroups, which could enable improved treatment targeting, faster response times, and improved cost savings.
Future Directions in Psoriasis Research
Professor Lars Iversen and Professor Ulrich Mrowietz
Disease Localisation and Memory
Current guidelines support individualisation of treatment based on the sites affected, with associated upgrade criteria for involvement of areas such as the face, genitals, and scalp.17,45 Evidence from patient interviews reflects that involvement of the scalp is an important area to treat quickly and effectively, as this is an area susceptible to scarring and the resulting dandruff can be upsetting for the patient.46 Psoriasis is known to be more difficult to treat in some areas of the body than others; for example, the scalp, face, genitals, lower legs, hands, feet, and nails are difficult-to-treat areas where residual psoriasis may be seen after the rest of the body is cleared.47,48 Although site may be a factor in the escalation of treatment, the authors’ expert
panel commented that, from its experience, a treatment shown in clinical trials to clear the main areas of the body can clear psoriasis in these difficult areas with comparable efficacy.
A current area of research focusses on determining the role of disease memory in psoriasis. Indicators of potential disease memory for psoriasis have been observed in patients for whom previously resolved lesions have returned in the same locations. A group of patients had the sites of their psoriasis photographed and recorded prior to travelling to the Dead Sea for therapy; those patients who achieved clear skin to the extent that they could stop all psoriasis treatment were asked to contact the clinic when they saw the first signs of new lesions. Upon re-photographing the patients, it was observed that the new lesions were not random and >60% of new lesions occurred at the same sites as previous lesions.49 The mechanism underlying disease memory in psoriasis is yet to be determined, but it could be related to a specific subtype of CD4+ and CD8+ T cells known as tissue-resident memory T cells.50
Disease Modification and Holistic Management
If disease memory has a significant role in psoriasis, preventing widespread development of the disease by identifying strategies for disease modification could be the key to limiting its impact. The concept of disease modification is more advanced in other immune-related conditions such as RA. In an RA study, patients who received 2 years of early intensive treatment with a combination of three disease-modifying antirheumatic drugs (methotrexate, sulfasalazine, and hydroxychloroquine with prednisolone) showed significantly lower levels of radiologic progression after 11 years compared to patients who received just sulfasalazine with or without prednisolone as the initial therapy.51
While future studies will assess if newer biologic agents have disease-modifying potential in psoriasis, clinicians must not neglect the opportunity to modify disease through management of risk factors and triggers. Obesity is an independent risk factor for psoriasis and has been shown to reduce treatment response.52 Psoriasis risk is increased in smokers and a dose-effect relationship has been documented.53
Common trigger factors related to psoriasis flare-ups include tonsillitis, periodontitis, and stress. Streptococcal throat infections and periodontitis are thought to share common pathways of T-cell stimulation and cytokine activation with psoriasis.54,55 Periodontitis risk is increased in patients with psoriasis and is related to psoriasis severity,56 whereas psoriasis has been shown to improve in patients following tonsillectomy.57 Psoriasis has been identified as one of the many diseases in which stress is a trigger factor. A study in new USA soldiers and soldiers with post-traumatic stress disorder showed a strong association between post-traumatic stress disorder and the genomic signatures for both psoriasis and RA.58
With an effective management of known risk and trigger factors, psoriasis disease modification is possible. Further research is required to define the impact of smoking cessation, stress prevention, and dental health on plaque psoriasis.







