Meeting Summary
In this article, the authors share and discuss data reported in three posters at the European Academy of Dermatology and Venereology (EADV) Congress held from 9th to 13th October 2019 in Madrid, Spain. Dimethyl fumarate (DMF) is a simple molecule derived from fumaric acid, which was approved as an oral monotherapy by the European Medicines Agency (EMA) in June 2017 for the treatment of adults with moderate-to-severe chronic plaque psoriasis. Two posters discuss preliminary results from an interim analysis on the efficacy and patient-reported outcomes (PRO) from the DIMESKIN 1 study, an open-label clinical trial to assess the long-term efficacy and safety of DMF treatment in adults with moderate-to-severe chronic plaque psoriasis (safety results will be analysed in depth in a final analysis at the end of the study). The first poster reports preliminary results on DMF efficacy over 24 weeks of treatment, observing comparable conditions to real-world clinical practice. The second presents preliminary results on PRO from DIMESKIN 1 at 24 weeks of treatment. A third poster reports pre-clinical study data on potential drug interactions with DMF and its primary active metabolite monomethyl fumarate (MMF).
Introduction
Psoriasis is associated with high morbidity, causing problems in daily life such as itching and scaling, even for patients with less-severe disease. Skin manifestations can lead to both emotional and physical distress in patients with psoriasis. Despite the wide variety of available treatments for psoriasis, the disease remains undertreated in some patients and there remains an unmet need for additional treatments.
Efficacy of Dimethyl Fumarate in Clinical Practice Among Patients with Moderate-to-Severe Plaque Psoriasis: Interim Analysis Through 24 Weeks from the DIMESKIN 1 Study1
Doctor Jose-Manuel Carrascosa
Psoriasis is a chronic inflammatory disease. Plaque psoriasis, the most common form of the disease, is characterised by red scaling plaque lesions which often cause discomfort including pain and itching to the patient, and impact quality of life (QoL).2-4 Psoriasis affects approximately 2–3% of the Western population,2,5 and is considered to be an immune-mediated disorder, although its aetiology is not yet fully understood. Psoriasis phenotype and pathogenesis is a result of a combination of genetic, environmental, and immunological factors.4,6 The disease pathogenesis is largely mediated by T cells and dendritic cells, with a proinflammatory cytokine network playing a central role.4,6
Fumaric acid esters (FAE) are lipophilic ester derivatives of fumaric acid that have demonstrated antipsoriatic efficacy over a number of decades and are mainly in use in Germany, but also in other European countries. This FAE preparation included DMF and a mixture of fumarate salts.4 DMF was subsequently recognised as the active component responsible for the antipsoriatic effects of this preparation,7 and is approved as an oral monotherapy for the treatment of adults with moderate-to-severe chronic plaque psoriasis.4,8,9 FAE have been recommended by European treatment guidelines as systemic therapy for both induction and long-term treatment of patients with moderate-to-severe chronic plaque psoriasis.10 As the last update of the S3 guidelines was published at the same time as the approval of DMF by the EMA, DMF has not yet been specifically discussed in treatment guidance. However, the published report from a 2018 expert consensus meeting on clinical use of DMF in moderate-to-severe plaque psoriasis offers guidance on appropriate patient selection, DMF dosage considerations, monitoring, and side-effect management.11 The mechanism of action of DMF is still being investigated, but is thought to be a result of a combination of biological effects; it is known to have anti-inflammatory properties, linked to promotion of the Th2 immune response.7
DIMESKIN 1 is an open-label clinical trial to assess the long-term efficacy and safety of DMF treatment in adults with moderate-to-severe chronic plaque psoriasis over a 52-week period, in 37 treatment centres across Spain.12
This poster reports the results of an interim analysis, 24 weeks into the DIMESKIN 1 trial. The objective was to assess DMF efficacy over 24 weeks of treatment in patients with moderate-to-severe plaque psoriasis, observing comparable conditions to real-world clinical practice, based on observed cases (OC) and last-observation-carried-forward (LOCF).
Adult patients with psoriasis were treated with DMF according to clinical practice, although some administration restrictions linked to the protocol should be taken into consideration. Efficacy analyses were performed on the intention-to-treat (ITT) population (≥1 post-baseline Psoriasis Area and Severity Index [PASI] value). Efficacy was assessed based on body surface area (BSA); PASI 50, 75, 90, and 100 response rates; absolute PASI scores ≤5, ≤3, and ≤1; and Physician’s Global Assessment (PGA) scores of 0 or 1 (‘clear’ or ‘almost clear’). Reported figures are only provided for OC and LOCF, because the interim analysis is based on these. Data on DMF efficacy in the ITT population will be published on completion of the final analysis for DIMESKIN 1.
A total of 175 patients were included in this analysis (73.1% male), with a mean age of 46.2 years (standard deviation [SD]: 13.1). Mean time since diagnosis was 17.1 (SD: 13.0) years, with median number of relapses in the previous year of 2 (range: 0–20). Most patients (83.4%) had previously received topical treatment, 40.6% had undergone phototherapy, and 60.0% had undergone systemic therapy. After 24 weeks of DMF treatment, median affected BSA showed a significant decrease from 15.0 to 2.0 in OC patients, and from 13.8 to 6.4 in LOCF patients (both p<0.001). Median absolute PASI also showed a significant decrease in both the OC and LOCF populations; from 12.3 to 2.0 and from 11.9 to 5.3, respectively (both p<0.001). PASI responses also increased over time, with 83.5% (OC) and 51.4% (LOCF) of patients achieving PASI 50 responses at Week 24, while 64.7% (OC) and 37.1% (LOCF) of patients achieved PASI 75 responses (n=85 for OC at 24 weeks; Figure 1).
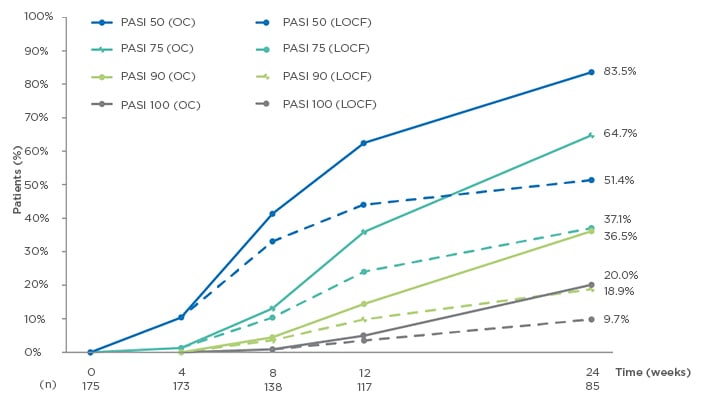
Figure 1: Evolution of Psoriasis Area Severity Index (PASI) 50/75/90/100 response rates from baseline to Week 24.1
LOCF: last observation carried forward (n=175); OC: observed cases; PASI: Psoriasis Area Severity Index.
Absolute PASI ≤5, ≤3, and ≤1 also increased over time; at Week 24, proportions of patients achieving PASI ≤5, ≤3, and ≤1 were 76.5%, 67.1%, and 31.8% of OC patients, respectively, and 48.6%, 40.6%, and 16.0% of LOCF patients, respectively. The proportion of patients with PGA assessed as clear or almost clear increased from 3.4% at Week 4 to 55.8% at Week 24 in the OC population (n=86 at 24 weeks), and from 3.4% to 33.1% at Week 24 in the LOCF population. The DMF safety profile was similar to that previously described with fumarates;9,13 adverse events in the safety population were mostly mild (63.3%) or moderate (31.7%), with the most common being gastrointestinal events, lymphopenia, and flushing. Safety data were not assessed in detail at this interim analysis; a full safety analysis will be published on completion of the study.
These preliminary data from the DIMESKIN 1 study at 24 weeks demonstrate a significant improvement with DMF therapy from baseline to 24 weeks, mainly in patients previously treated with topical, systemic, or phototherapy. Patients showed improvement in all major measures of efficacy assessed (BSA, PASI, PGA), with safety findings comparable to previous studies, and a notable improvement was observed as early as Week 8 of treatment. A strength of the DIMESKIN 1 study is that it provides the first long-term interim data on DMF treatment, reporting at 24 weeks (as part of a 1-year study). Limitations include the current interim analysis status of the study data, meaning that these data may vary compared with the final analysis. A detailed safety analysis was also not part of the interim analysis; therefore, discussion of safety outcomes can only be limited at this stage.
Improvement of Patient-Reported Outcomes in Patients with Moderate-To-Severe Plaque Psoriasis on Dimethyl Fumarate Treatment: Interim Analysis Through 24 Weeks from the DIMESKIN 1 Study14
Doctor Jose-Manuel Carrascosa
Psoriasis is associated with high morbidity. Skin manifestations often cause patient anxiety and embarrassment, and can lead to both emotional and physical distress.2,3,6,15 The USA National Psoriasis Foundation (NPF) survey (2003–2011) found that psoriasis and psoriatic arthritis affected emotional wellbeing in 88% of patients, with 82% reporting that their disease interfered with their enjoyment of life.16 Psoriasis therefore has a major effect on the lives of patients with even minimal disease manifestations, while medication-associated side-effects can also affect patient QoL.2,3,6
Psoriasis-associated morbidity can lead to negative effects on mental functions.3,16 Greater psoriasis severity is associated with poorer QoL;3,17 in a real-world setting, more-severe psoriasis was associated with worse PRO, measured by Dermatology Life Quality Index (DLQI) and visual analog scale (VAS) assessment.17 Although PRO are subjective, they can be an important measure of how patients are coping with their disease and can provide insight into patient experiences with the healthcare that they receive, as well as an indication of suboptimal disease control.18 Furthermore, patient satisfaction with therapy can improve adherence, supporting improved long-term patient outcomes.19
This poster reports PRO at a 24-week interim analysis into the DIMESKIN 1 study, assessing DMF treatment impact in adult patients with psoriasis. As noted previously, the analysis was in the ITT population and was based on OC and LOCF. PRO such as the DLQI questionnaire and VAS assessment were evaluated to quantify pruritus and to measure patient satisfaction with treatment. The patient population and demographics are the same as reported in the previous poster.
The DLQI is a dermatology-specific tool to measure health-related QoL. Respondents are asked to answer 10 questions within the domains of symptoms and feelings, activities (daily and leisure), work or school, personal relationships, and treatment. They are asked to show the degree that they feel they have experienced problems over a period of 1 week, and a 4-point Likert scale (from 0 meaning not at all, to 3 meaning very much) is used to assess their responses. A total DLQI score of 0–30 is then calculated, with higher scores showing worse QoL. A score of ≤10 on the DLQI is normally considered to denote mild disease, while a score of >10 demonstrates notable impact on QoL, with the need to consider systemic therapy. The therapeutic target is usually DLQI ≤5 during maintenance treatment; a score of >5 suggests a need for modification of the treatment regimen.17,20 The VAS is a self-reported health scale scored from 0 to 10 (with 0 being ‘best imaginable health status’ and 10 being ‘worst imaginable health status’). The VAS can also be used specifically to assess pruritus.21,22
At Week 24 of the study, median DLQI scores had significantly decreased, from 10.5 (OC) and 11.0 (LOCF) at baseline, to 1.0 (OC) and 3.0 (LOCF) at Week 24 (both p<0.001); also, the proportion of patients with DLQI scores of ≤5 and ≤1 increased from baseline to 24 weeks. DLQI scores of ≤5 were seen in 79.8% (OC) and 61.7% (LOCF) of patients, and of 0–1 in 54.8% (OC) and 34.9% (LOCF) of patients at 24 weeks (OC: n=84; Figure 2).
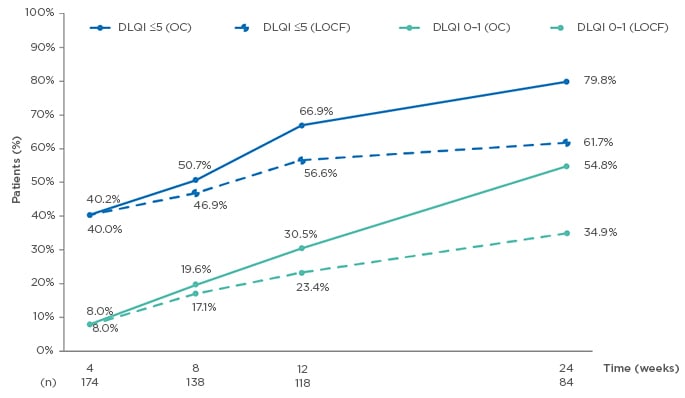
Figure 2: Evolution of Dermatology Life Quality Index (DLQI) ≤5 and DLQI 0–1 from baseline to Week 24.14
DLQI: Dermatology Life Quality Index; LOCF: last observation carried forward (n=175); OC: observed cases.
Median pruritus VAS scores significantly decreased in the OC population after 24 weeks of DMF treatment (from 7.0 to 1.5 OC; p<0.001); the proportion of patients without pruritus (VAS=0) increased in the OC population from 2.3% at baseline, to 31.4% at 24 weeks (n=86; Figure 3). The distribution of patient satisfaction with treatment by VAS at Week 24 demonstrated mostly high scores for satisfaction with DMF on the scale of 0–10 points (with 10 as maximum satisfaction), with 37.2% of patients giving a score of 10, and 16.7% each scoring 9 and 8; 18% of the patients reported a satisfaction score between 5 and 7, and 11.6% of patients reported a score <5. Median patient satisfaction with treatment was 9 points (OC: n=78).
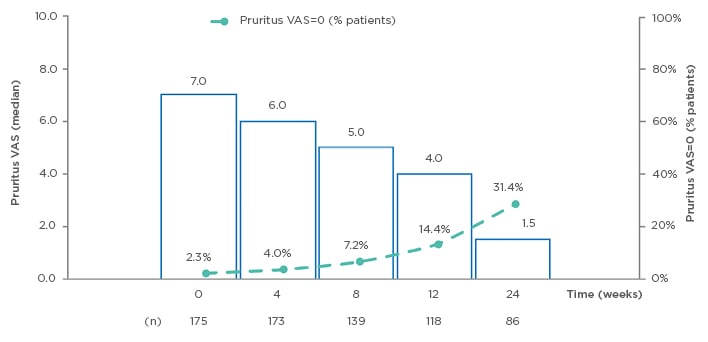
Figure 3: Evolution of pruritus visual analog scale (VAS) (observed cases) from baseline to Week 24.14
VAS: visual analog scale.
Preliminary data from the DIMESKIN 1 study at 24 weeks therefore suggest a significant improvement in pruritus at early stages (as measured by VAS; p<0.001) and QoL in patients with psoriasis (p<0.001), beginning from Week 8 as measured using the DLQI questionnaire. Furthermore, patient satisfaction with DMF was high, with the majority of patients (70.6%) scoring 8–10 on the VAS for patient satisfaction (median: 9 [OC]).
No Evidence for Interactions of Dimethyl Fumarate and its Main Metabolite Monomethyl Fumarate with Human Cytochrome P450 Enzymes and the P-Glycoprotein Transport System23
Doctor Jordi Aubets
Psoriasis has been linked to an increased comorbidity presence, which may include diabetes and cardiovascular, liver, and renal disease, with a dose-dependent relationship between psoriasis disease severity and these comorbidities.24 There is also evidence linking psoriasis to the metabolic syndrome.25 Drug–drug interactions are an important consideration in managing treatment, particularly in those patients who may have comorbidities, requiring a multiple drug regimen (polypharmacy) which can lead to potentially harmful combinations of drugs.26 Drug–drug interactions are thought to significantly contribute to the onset of adverse drug events in patients needing polypharmacy.27 Comorbidity presence and an existing drug regimen are therefore both important considerations when selecting treatment in patients with psoriasis.24,28
In vivo inhibition of cytochrome P450 (CYP) enzymes occurs with a large variety of drugs (e.g., midazolam and ketoconazole), affecting the metabolic disposition of any co-administered drugs that are also metabolised by these enzymes.29 The P-glycoprotein (P-gp) efflux membrane transporter is responsible for limiting cellular uptake, and for extruding a wide range of structurally diverse compounds from the cell. It is widely distributed throughout the body, and is mainly found in epithelial cells with excretory roles.30 The effect of P-gp action is to limit oral absorption and brain penetration.31 New treatment safety assessments should include investigations on the potential for pharmacokinetic interactions between drugs, covering both the potential impact of the investigational drug on other medicinal products, and the effects of other drugs on the investigational drug. These investigations should include enzymes that are heavily involved in drug metabolism (i.e., the CYP enzymes), and proteins involved in drug transport and elimination, hence the investigation of P-gp. The majority of clinically significant drug–drug interactions are caused by drug interaction with the CYP enzymes. Based on this, both the EMA and U.S. Food and Drug Administration (FDA) recommend assessment of CYP enzyme inhibition as an integral part of drug safety assessment. Several drugs are selected as a positive control for these studies, and the results are then extrapolated in relation to other drugs that are metabolised or transported by the same systems.32-34 This poster reports the results of in vitro studies assessing potential interactions of DMF and its primary, active metabolite MMF, with CYP and P-gp.
CYP-selective substrates were added to human liver microsomes, following DMF or MMF incubation. Metabolite formation was measured using liquid chromatography with tandem mass spectrometry. No inhibition of CYP3A enzymes was demonstrated by DMF at concentrations up to 666 μM, or by MMF at concentrations up to 750 μM. Concentrations that produced a half maximal inhibitory concentration (IC50) inhibition could not be determined for DMF or MMF; therefore, inferred IC50 values were >666 μM and >750 μM for DMF and MMF, respectively.
MMF effects on CYP mRNA expression were also assessed in cryopreserved human hepatocytes, following a 72-hour exposure period. Increases were seen in CYP1A2 and in CYP2B6 mRNA expression at 250 μM MMF in donor 4 (this concentration is 22 times greater than the clinically relevant maximum plasma concentration of MMF of 11.2 μM for a 240 mg dose);35 a >2-fold increase in CYP3A4 mRNA was also seen in donor 2, but was not concentration dependent, and was not replicated in donors 3 or 4.
DMF and MMF absorption were predicted based on apparent permeability (Papp) in Caucasian colon adenocarcinoma (Caco-2) cells. DMF permeability across Caco-2 cell monolayers was concentration-dependent at 120 minutes; moderate-to-high (Papp: ≥2.3–29.7×10-6 cm/s) cell permeability was demonstrated by DMF in both A–B and B–A directions. MMF permeability in the Caco-2 system was low-to-moderate in both A–B and B–A directions (Papp: 1.2–8.9×10-6 cm/s at 0.0738–0.738 mM; undetermined at 7.380 mM).
The potential for DMF and MMF to act as P-gp substrates was assessed in Madin–Darby Canine Kidney (MDCKII) cells transfected with the human P-gp gene; inhibitory P-gp interactions of DMF and MMF were assessed by incubation with [3H]digoxin in Caco-2 and in MDCKII cells, and the bidirectional transport of [3H]digoxin was measured. The study objectives were to assess the effect of these enzymes and transporters on the oral absorption of DMF prior to marketing. The studies conducted were based on regulatory requirements, and the cell lines used are those standardised between pharmaceutical companies to allow easy comparison between studies.32-34 Incubation data from MDCKII cells suggested that DMF and MMF were not P-gp substrates, but incubation in Caco-2 cells suggested weak DMF inhibition of P-gp. MMF was not found to be an inhibitor of P-gp. The IC50 values for DMF were 1.5 mM and 0.9 mM in Caco-2 and MDCKII cells, respectively.
These in vitro study results provided no evidence to suggest direct inhibition of CYP enzymes by DMF or MMF at clinically relevant concentrations (the reported IC50 values for DMF and MMF of >666 μM and >750 μM, respectively, would not be reached in clinical practice). Furthermore, MMF did not induce CYP enzyme mRNA expression at clinically relevant concentrations. DMF is likely to be a weak inhibitor of P-gp, but as it is rapidly hydrolysed to MMF in the gastrointestinal tract, and because the DMF IC50 is high (in the mM range)36,37 this is not expected to be of clinical relevance.
No interactions are therefore predicted between DMF or MMF and medicinal products metabolised or transported by the CYP or P-gp systems, respectively, at clinically relevant concentrations.
The potential for complex multiple drug regimens in patients with psoriasis means that it is important to minimise the risk of drug–drug interactions when selecting therapy. These preclinical study results suggest that DMF is unlikely to cause drug–drug interactions in patients with psoriasis. By contrast, ciclosporin is an inhibitor of CYP3A4, P-gp, and organic anion transporter proteins. Therefore, ciclosporin is contraindicated for use in combination with medicines that are substrates of CYP3A4, P-gp, or organic anion transporter proteins. Caution and increased monitoring are advised in the concomitant use of ciclosporin with drugs that are inducers of CYP3A4 or P-gp.38
SUMMARY
In summary, DMF is an oral systemic therapy for the treatment of adults with moderate-to-severe chronic plaque psoriasis.4,9 The two posters reporting interim data from the DIMESKIN 1 study provide evidence to support the efficacy of DMF as a systemic therapy for moderate-to-severe psoriasis, with improvement in patient-reported outcomes, satisfaction, and QoL. A full analysis of the safety data will be published on completion of the DIMESKIN 1 study. Overall, results from the third poster presented showed no evidence to suggest that DMF or MMF interact with CYP enzymes or P-gp at clinically relevant concentrations; no interactions are therefore predicted between DMF and medicinal products metabolised or transported by these systems.







