Meeting Summary
Nemolizumab is an anti-IL-31Rα monoclonal antibody that has demonstrated clinical efficacy in two global Phase III studies (ARCADIA-1 and ARCADIA-2) in adults and adolescents with moderate-to-severe atopic dermatitis (AD). This article summarises selected data from oral presentations on nemolizumab at the European Academy of Dermatology and Venereology (EADV) Annual Meeting held in September 2024 in Amsterdam, the Netherlands.
Data from interim analyses were presented from the ARCADIA open-label extension study that evaluated the long-term safety and efficacy of nemolizumab in adults and adolescents with moderate-to-severe AD. These first long-term data on nemolizumab treatment showed continuous improvement up to 56 weeks in key signs and symptoms of AD, as well as patient-reported quality of life (QoL) measures. Safety findings were consistent with those previously reported, further supporting the long-term use of nemolizumab. Maintaining long-term efficacy and safety over extended periods of treatment is crucial in the management of chronic skin diseases, such as AD, which impose a substantial patient burden.
The second presentation demonstrated how clinical improvements in signs and symptoms of AD with nemolizumab treatment correlate with a notable impact on pruritus and hyperplasia/fibrosis biomarkers in the skin. Tape-strips transcriptomic analysis was used to evaluate the effect of nemolizumab on gene expression in a subset of patients with AD from the ARCADIA 1 and 2 studies. Results showed that nemolizumab robustly modulates gene expression, acting on biomarkers related to the three key pillars of AD pathogenesis: pruritus, inflammation, and skin barrier dysfunction. Importantly, patients with severe pruritus at baseline showed stronger downregulation of inflammatory markers, including Type 2, Type 17, and Type 1 biomarkers, highlighting nemolizumab’s therapeutic potential in patients with AD extensively affected by itch.
Introduction
AD is a common, chronic, inflammatory skin condition that requires long-term treatment.1,2 The hallmark clinical symptom of AD is pruritus (itch), which significantly impacts patients’ QoL.3
Nemolizumab is an IL-31Rα antagonist that inhibits the binding of IL-31, a neuroimmune cytokine and key mediator for itch in AD, to its receptor.4-6 IL-31 signals through a heterodimeric receptor composed of OSMRβ and IL-31Rα, triggering itch, skin barrier disruption, and exacerbation of inflammation, and is strongly implicated in the pathogenesis of AD.6-8
Nemolizumab was evaluated in two global Phase III studies, ARCADIA-1 (NCT03985943) and ARCADIA-2 (NCT03989349), in adults and adolescents with moderate-to-severe AD, in which it provided clinically meaningful improvements in itch, skin lesions, and sleep at Week 16 and responses were sustained up to Week 48.9
Nemolizumab Long-Term Safety and Efficacy up to 56 weeks in the ARCADIA Open-Label Extension Study in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis
Diamant Thaçi from the Institute and Comprehensive Centre for Inflammatory Medicine, University of Lübeck, Germany, presented interim data up to 56 weeks from the ARCADIA long-term extension study, which aimed to evaluate the long-term safety and efficacy of nemolizumab up to 200 weeks in patients aged ≥12 years with moderate-to-severe AD.10
The population for this prospective, multicentre, open-label, long-term extension (LTE) study (NCT03989206) consisted of patients from lead-in Phase II/IIb and Phase III/IIIb studies (including the ARCADIA 1 and 2 trials), as well as newly recruited adolescents.11 As Thaçi explained, this resulted in a heterogeneous baseline patient population. Patients received 30 mg subcutaneous nemolizumab every 4 weeks up to Week 200 on a background of low/medium potency topical corticosteroids with or without topical calcineurin inhibitors.
Data from patients in the LTE were analysed according to the treatment received in the lead-in studies: those with previous nemolizumab experience (PNE; n=962) and those who were nemolizumab naïve (NN; n=536). A total of 723 patients (41.6%) had completed Week 56 at the data cut-off (September 30 2022). The overall discontinuation rate for the PNE and NN cohorts in the LTE study was 21.6% and 25.3%, respectively, with the main reason for discontinuing treatment being patients’ own request.
Thaçi presented data as observed from evaluable patients at each time point up to 56 weeks in the nemolizumab LTE study across a variety of key AD efficacy outcome measures. Ongoing and clinically meaningful improvements in skin lesions were observed through Week 56 as measured by an Investigator’s Global Assessment (IGA) score of 0/1 (clear/almost clear) and Eczema Area and Severity Index (EASI) 75 scores. Notably, the response to treatment in the NN group also converged to treatment response in the PNE group over 56 weeks.
At Week 56, 47% and 49% of PNE and NN evaluable patients achieved IGA 0/1, respectively, with long-term nemolizumab treatment, from baseline levels of 29% and 18%. Similarly, the proportion of PNE and NN evaluable patients attaining EASI-75 increased from 38% and 24%, respectively, at baseline to 73% and 79% after 56 weeks of nemolizumab treatment (Figure 1).
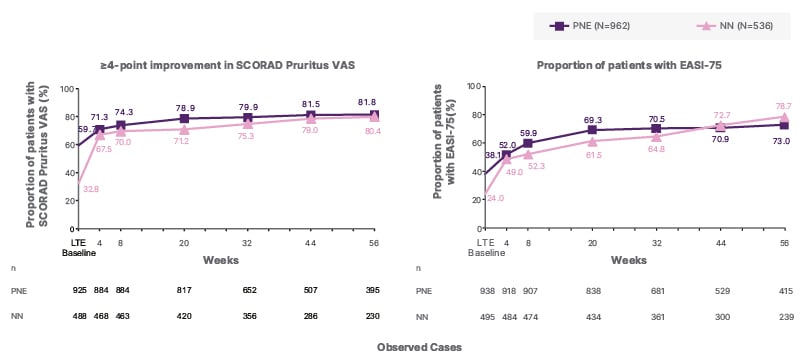
Figure 1: Proportion of evaluable patients with IGA score of 0/1 and EASI-75 up to 56 weeks.
Analysis was conducted in safety population, defined as patients who received at least one dose of the study drug. The endpoint was summarised using observed cases (OC analysis). LTE baseline is the last available measurement prior to the first treatment in this study.
EASI: Eczema Area and Severity Index; EASI-75: 75% improvement in EASI from lead-in baseline; IGA: Investigator’s Global Assessment; LTE: long-term extension; N: total number of patients in the safety population; n: number of patients with available data; NN: nemolizumab-naïve; PNE: previous nemolizumab experience; SCORAD: SCORing Atopic Dermatitis; VAS: visual analogue scale.
Moreover, improvements in itch and sleep were observed over time with long-term nemolizumab treatment as measured by changes in the SCORing Atopic Dermatitis (SCORAD) score, including itch and sleep visual analogue scale (VAS) components (Figure 1). SCORAD Pruritus VAS improved by approximately 75% and SCORAD Sleep Loss VAS by about 70%, versus lead-in baseline, in both NN and PNE groups after 56 weeks of treatment.
In addition, patient-reported outcomes such as QoL, as measured by the Dermatology Life Quality Index (DLQI), continued to improve over 56 weeks of nemolizumab treatment. Overall, 86% of PNE and 90% of NN evaluable patients experienced a ≥4 point reduction in DLQI by Week 56, from LTE baselines of 77% and 63%, respectively. The proportion of evaluable patients achieving DLQI 0/1 at Week 56 was 38% and 43% in the PNE and NN cohorts, respectively, compared to LTE baselines of 27% and 14%.
Safety data from the ARCADIA LTE study were reassuring and supported the long-term use of nemolizumab for the treatment of adolescent and adult patients with moderate-to-severe AD. Adverse events (AE) were consistent with those previously reported (Table 1).10 COVID-19 and AD were the most common treatment-emergent AEs (TEAE) occurring in ≥5% of patients.
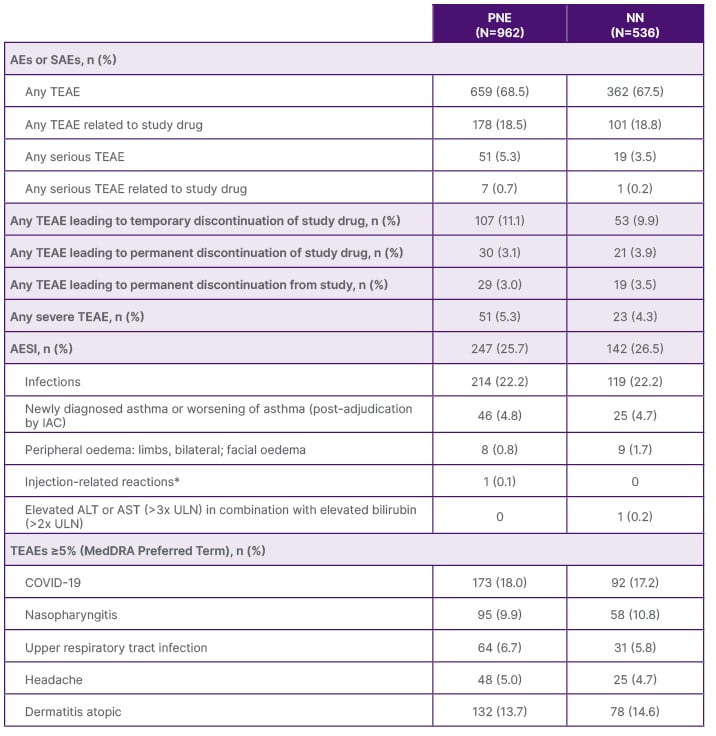
Table 1: Overall summary of treatment-emergent adverse events during treatment period.
*Injection-related reactions include anaphylactic reactions, acute allergic reactions requiring treatment, and severe injection site reactions with a duration of >24 hours.
AE: adverse event; AESI: adverse event of special interest; ALT: alanine transaminase; AST: aspartate transaminase; IAC: independent adjudication committee; MedDRA: Medical Dictionary for Regulatory Activities; N: total number of patients in treatment group in the safety population; n: number of patients with available data; NN: nemolizumab naïve; PNE: previous nemolizumab experience; SAE: serious adverse event; TEAE: treatment-emergent adverse event; ULN: upper limit of normal.
Overall, Thaçi concluded that these data from interim analysis of the ARCADIA LTE study support the long-term safety and efficacy of nemolizumab in patients with moderate-to-severe AD. Nemolizumab proved well-tolerated up to Week 56, and clinically meaningful improvements in AD signs and symptoms were observed with continuous treatment.
Tape-Strips Transcriptomic Analysis of Patients with Moderate-to-Severe AD Treated with Nemolizumab
Emma Guttman-Yassky from the Icahn School of Medicine at Mount Sinai, New York, USA, presented results from a biomarker sub-study that used tape-stripping to evaluate the effect of nemolizumab on the cutaneous transcriptomic profile in a subset of AD patients from the ARCADIA 1 and 2 studies.12
Tape-strips transcriptomic analysis, a minimally invasive method that provides a detailed molecular profile of the skin affected by AD,13 was used to study gene expression changes before and after treatment with nemolizumab.12 A total of 72 patients were treated with nemolizumab and 39 with placebo, and they were evaluated at baseline and after 16 weeks. No significant differences were observed between the two groups in terms of demographics or baseline characteristics. Tape-strips were collected from lesional and non-lesional skin of these patients. Transcriptomic analysis revealed significant gene changes during nemolizumab treatment. Notably, nemolizumab showed robust modulation of biomarkers of pruritus (e.g., TRPM1, TRPV3, and OSMR) and hyperplasia/fibrosis (e.g., KRT6C, SERPINB, COL1A1, COL12A1, IGFBP3, and SOX9). Nemolizumab also downregulated key immune markers, demonstrating improvement in both Th17/Th22 (S100A7, S100A8, S100A9, S100A12, IL36G, and PI3) and Th1 markers (e.g., CCL2, MX1, and OASL) across all patients.
Analysis of a subset of nemolizumab-treated patients with severe baseline pruritus (Peak Pruritus Numerical Rating Scale [PPNRS] >7) showed greater Th1, Th2, Th22/IL22, and Th17 immune activation at baseline and more robust immune downregulation upon nemolizumab treatment at Week 16 than in those treated with placebo. Specifically, patients with more severe itching showed stronger downregulation of Th2 (IL4R, CCL17), Th1 (CCL3/4, CXCL8), and Th17 (CXCL1, IL17RA, PI3).
Importantly, improvements in clinical efficacy parameters following nemolizumab treatment were found to be significantly positively correlated with these changes in fibrosis and pruritus markers. Specifically, improvements in EASI and PPNRS scores correlated significantly with the changes in Th17, fibrosis, skin barrier, pruritus, and lipid biomarkers amongst all nemolizumab-treated patients and those with severe itch at baseline (Figure 2).
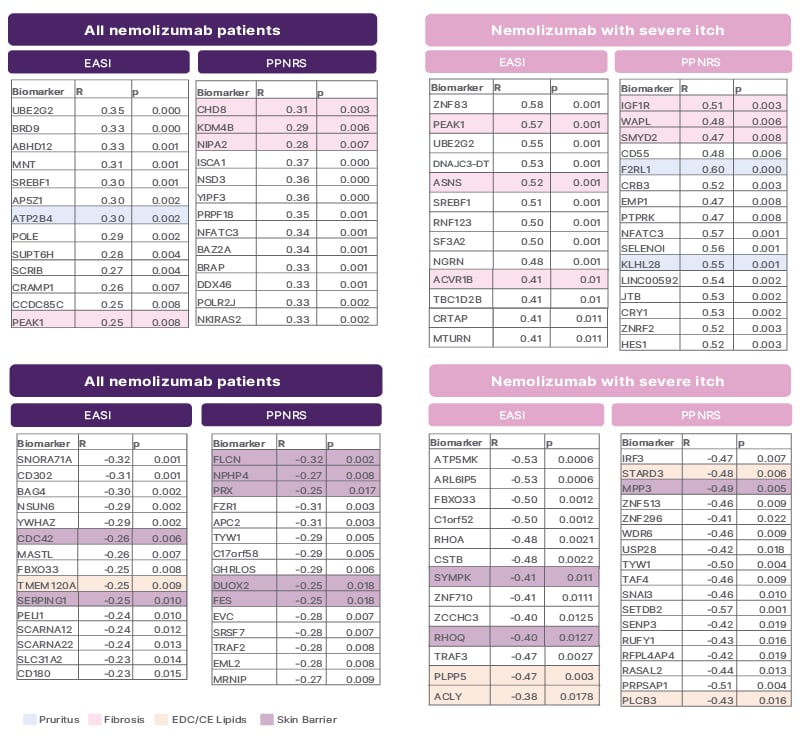
Figure 2: Improvements in Eczema Area and Severity Index and Peak Pruritus Numerical Rating Scale following nemolizumab correlate with changes in fibrosis, pruritus endocrine disrupting chemicals/cholesterol esters lipids, and skin barrier markers.
EASI: Eczema Area and Severity Index; PPNRS: Peak Pruritus Numerical Rating Scale.
Overall, these study findings show that nemolizumab exerts a robust impact on pruritus and hyperplasia/fibrosis biomarkers which correlate with clinical improvements in itch, skin, and QoL in patients with AD. The greater immune modulation in patients with severe pruritus highlights nemolizumab’s potential in patients with AD heavily impacted by itch, Guttman-Yassky concluded.






