Meeting Summary
IL-23 plays a key regulatory role in psoriasis, and the high-affinity anti-IL-23p19 monoclonal antibody tildrakizumab is effective for the treatment of adults with moderate-to-severe plaque psoriasis. At the meeting, two posters and an oral presentation explored the safety of tildrakizumab up to 148 weeks, including analyses of extended major adverse cardiovascular events (MACE), severe infections, and malignancies. Safety was assessed over extension periods of two Phase III, double-blinded, three-part, parallel group, randomised, placebo-controlled trials: reSURFACE 1 (N=772) and reSURFACE 2 (N=1,090) in adults with moderate-to-severe plaque psoriasis. Treatment switching occurred during the trial and the number of patients taking each treatment were 200 mg tildrakizumab (TIL 200; n=928), 100 mg tildrakizumab (TIL 100; n=872), placebo (n=543), or etanercept (reSURFACE 2 only) (n=313). Exposure-adjusted incidence rates (EAIR) (all preferred terms) are reported: any event ≥0.10 events/100 patient-years of exposure for severe infections; events/100 patient-years of exposure for MACE and malignancies. EAIR for MACE with TIL 200, TIL 100, etanercept, and placebo were 0.54, 0.40, 0.65, and 0.49, respectively. For severe infections, these were 1.12 (TIL 200), 1.14 (TIL 100), 1.96 (etanercept), and 0.97 (placebo). EAIR for malignancies, excluding non-melanoma skin cancer (NMSC), were 0.39, 0.55, 1.30, and 0.00 for TIL 200, TIL 100, etanercept, and placebo groups, respectively. For NMSC, respective EAIR were 0.49, 0.50, 1.30, and 0.97. In conclusion, in adults with moderate-to-severe plaque psoriasis there were low rates of MACE, severe infections, and malignancies in participants who had taken tildrakizumab over 148 weeks, comparable to the rates for etanercept and placebo.
Overview
IL-23 plays a key regulatory role in the pathogenesis of plaque psoriasis and medications targeting IL-23 have been shown to be effective for people with this skin condition.1–6 Tildrakizumab is a high-affinity, anti-IL-23p19 monoclonal antibody approved for treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.1
Trials showing efficacy and safety1,2,7 of tildrakizumab up to 64 weeks have been published, and long-term trials have been carried out for up to 3 years. The oral presentation and two posters detailed at the meeting explored several safety aspects of tildrakizumab over 148 weeks, including analyses of confirmed extended MACE, severe infections, and malignancies.
Study Design
Tildrakizumab safety and efficacy were assessed following the initial study and extension periods of two Phase III, double-blinded, three-part, parallel-group, randomised, placebo-controlled trials: reSURFACE 1 and reSURFACE 2
(Figure 1). Extension period efficacy results are not discussed here. These studies took place over numerous sites, including hospital dermatology units, speciality clinics, private practices, and research centres in several European countries, North America, Australasia, and Asia.1,2
In Part 1, adults with moderate-to-severe plaque psoriasis were randomised to TIL 200, TIL 100, a placebo, or etanercept (reSURFACE 2 only) (Figure 1). In Part 2, the placebo arm was switched to TIL 100 or TIL 200 after Week 12. From Week 28, treatment in Part 3 was determined based on treatment in the previous arms and treatment response as assessed by Psoriasis Area and Severity Index (PASI) and Physician’s Global Assessment (PGA) scores1,2 (Figure 1). Etanercept responders were discontinued in Part 3; non or partial responders (PASI ≥50) were switched to TIL 200.
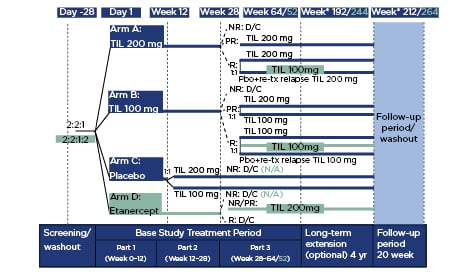
Figure 1: Study design.
Differences in design for reSURFACE 2 versus reSURFACE 1 are shown in turquoise. *Wk 192/212 is starting at extension Week 0.
D/C: discontinued; N/A: not applicable; NR: non-responders; Pbo: placebo; PR: partial responders; R: responders; TIL: tildrakizumab; tx; treatment.
Adapted from Reich et al.1
Overall participant numbers in the trials were 772 in reSURFACE 1 and 1,090 in reSURFACE 2.1,2 For the analysis, each group included those who received treatment in at least one part of the study. Some participants were switched between treatments during the trials and they received more than one treatment; therefore, the numbers in each treatment group are higher than the participant numbers. Treatment group numbers were 928 (TIL 200), 872 (TIL 100), 313 (etanercept), and 543 (placebo). Total patient-years of follow-up were 2,046.71 (TIL 200), 2,014.49 (TIL 100), 153.42 (etanercept), and 205.30 (placebo). An adverse event (AE) was assigned to the treatment taken at the time the AE occurred, e.g., if an AE happened while a participant was in a TIL group, the AE was assigned to this group, even if they had initially received etanercept.
Groups were well balanced according to baseline characteristics that were considered reasonably standard for this population. Mean age ranged from 45.7 to 46.6 years (standard deviation: 12.6–14.0), mean weight ranged from 88.0 to 89.1 kg (21.5–23.6), and the majority in all groups were male (70–72%). Mean PASI and mean affected body surface area scores were also similar, ranging from 19.8 to 20.2 (7.4–7.9) and 29.8 to 31.8% (16.6–17.8), respectively.
For severe infections, EAIR of any event ≥0.10 events per 100 patient-years of exposure were reported. For MACE and malignancies, events/100 patient-years of exposure were reported (Table 1).
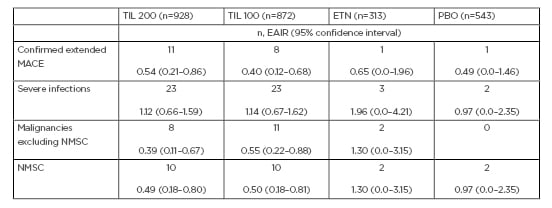
Table 1: Exposure-adjusted incidence rates (all preferred terms).
Data are n, events per 100 patient-years of exposure (95% confidence interval) for MACE and malignancies (NMSC and non-NMSC). Severe infections were considered ≥0.10 events per 100 patient-years of exposure (95% confidence interval).
EAIR: exposure-adjusted incidence rates; ETN: etanercept; MACE: major adverse cardiovascular event; NMSC: non-melanoma skin cancer; PBO: placebo; TIL: tildrakizumab.
Incidence of Confirmed Extended Major Adverse Cardiovascular Events
Oral Presentation by Doctor Ignasi Pau-Charles (lead Author: Doctor Lars Iversen)
People with psoriasis are at increased risk of cardiovascular events (CE).8-10 While the usual definition of major CE includes only non-fatal myocardial infarction, non-fatal stroke, and cardiovascular deaths, in this analysis ‘extended MACE’ also included unstable angina, coronary revascularisation, resuscitated cardiac arrest, and cardiovascular deaths confirmed as ‘cardiovascular’ or ‘sudden’. Deaths and serious CE were adjudicated by an external clinical committee.
Notably, around 25% of the participants had a history of at least one CE or disease and the most prevalent risk factor was hypertension, occurring in 26.0–28.1% of the participants. Other risk factors included hypercholesterolaemia, hyperlipidaemia, obesity, and Type 2 diabetes mellitus, all with prevalences of 4–8%, with a lower prevalence of hypertriglyceridaemia (0.6%).
In the TIL 200 and TIL 100 groups, there were 0.54 and 0.40 events of confirmed extended MACE per 100 patient years, respectively (i.e., EAIR) (see Table 1 for incidences and 95% confidence intervals [CI]). The events with the highest EAIR were coronary artery disease (EAIR: 0.20; CI: 0.0–0.39/four events in the TIL 200 group) and acute myocardial infarction (EAIR: 0.15; CI: 0.00–0.32/3 in the TIL 200 group, EAIR: 0.50; CI: 0.00–15/1 in the TIL 100 group).
The EAIR was 0.05 (CI: 0.00–0.15/1) with TIL 200 for aneurysm, angina pectoris, coronary artery stenosis, myocardial infarction, and transient ischaemic attack, and with TIL 100 for angina pectoris, cerebral haemorrhage, cerebral infarction, cardiovascular accident, death, myocardial infarction, respiratory arrest, and thrombotic cerebral infarction. There was one reported case of MACE each in the etanercept and placebo groups (coronary artery stenosis, EAIR: 0.65; CI: 0.00–1.96, and cerebellar infarction, EAIR: 0.49; CI: 0.00–1.46, respectively), meaning the overall EAIR in both groups were comparable to those in the TIL groups (Table 1).
Four deaths were recorded, all in those with a history of cardiovascular risk factors. These included single cases of aneurysm (TIL 200), myocardial infarction (TIL 100), and respiratory arrest (TIL 100), and one (recorded as ‘death’) that was a presumed myocardial infarction (TIL 100), but with no further details provided. All were adjudicated as unrelated to study medication.
In conclusion, compared to both etanercept and placebo, tildrakizumab has a favourable long-term safety profile regarding confirmed extended MACE.
Incidence of Severe Infections
Poster Presented by Professor Diamant Thaçi (Poster No: 1954)
Some immune system-targeting therapies have been associated with severe infections,11-13 making it important to monitor over time. Here, severe infections were defined as any infection meeting the regulatory definition of a serious AE (SAE) or requiring intravenous antibiotics, irrespective of whether it was deemed a SAE.7
Overall, there was an EAIR of severe infections of 1.14 in the TIL 100 group and 1.12 in the TIL 200 group, with a higher value of 1.96 for the etanercept group and a slightly lower one of 0.97 for the placebo group (Table 1). Most commonly reported severe infections with TIL 200 were cellulitis (EAIR: 0.20; CI: 0.00–0.39/4), diverticulitis and pneumonia (both EAIR: 0.15; CI: 0.00–0.32/3), with an EAIR of 0.05 (CI: 0.00–0.15/1) for the following: appendicitis, gastroenteritis, herpes zoster, and wound infection. With TIL 100, the most commonly reported severe infections were appendicitis, cellulitis, diverticulitis, gastroenteritis, and sinusitis (all EAIR: 0.15; CI: 0.00–0.32/3), with an EAIR of 0.10 (CI: 0.00–0.24/2) for wound infection and 0.05 (CI: 0.00–0.15/1) for pneumonia. In the etanercept group, cellulitis, herpes zoster, and urosepsis all had EAIR of 0.65 (CI: 0.00–1.96/1), with an EAIR for cellulitis of 0.97 (CI: 0.00–2.35/2) in the placebo group.
These data indicate that the risk of severe infection is low in patients with moderate-to-severe plaque psoriasis being treated long term with tildrakizumab.
Incidence of Malignancies
Poster Presented by Doctor Kristian Reich (Poster No: 1975)
There is an increased risk of certain malignancies, including NMSC, in individuals with psoriasis.14 In these studies, the EAIR for malignancies (excluding NMSC) was highest in the etanercept group (1.30), with lower rates of 0.55 and 0.39 in the TIL 100 and TIL 200 groups, respectively. There were no malignancies reported in the placebo group (Table 1).
For TIL 200, the most frequent malignancy was pancreatic cancer (EAIR: 0.10; CI: 0.00–0.24/2). In both TIL groups, bladder transitional cell carcinoma, breast cancer, papillary thyroid cancer, and rectal adenocarcinoma had EAIR of 0.05 (CI: 0.00–0.15/1) , with the same values for metastatic breast cancer and prostate cancer in the TIL 200 group and for acute myeloid leukaemia, diffuse large B-cell carcinoma, leiomyosarcoma, malignant melanoma in situ, non-Hodgkin’s lymphoma, ovarian cancer, and thyroid cancer in the TIL 100 group. In the etanercept group, EAIR were 0.65 (CI: 0.00–1.96/1 ) for both breast cancer and lung adenocarcinoma.
The highest EAIR for NMSC was 1.30 in the etanercept group. The EAIR in the other groups were placebo (0.97), TIL 100 (0.50), and TIL 200 (0.49) (Table 1). The most frequent NMSC was basal cell carcinoma, with EAIR of 0.34 (CI: 0.08–0.60/7) with TIL 200, 0.30 (CI: 0.05–0.54/6) with TIL 100, 1.30 (CI: 0.00–3.15/2) with etanercept, and 0.49 (CI: 0.00–1.46/1) with the placebo. The second most frequent NMSC was squamous cell carcinoma of the skin: TIL 200: 2.0 (CI: 0.00–0.24/2); TIL 100: 0.15 (CI: 0.00–0.32/3); placebo: 0.49 (CI: 0.00–1.46/1). Bowen’s disease occurred with an EAIR of 0.05 (CI: 0.00–0.15/1) in the TIL 200 group and 0.10 (CI: 0.00–0.24/2) in the TIL 100 group, with an EAIR of 0.05 (CI: 0.00–0.15/1) for squamous cell carcinoma in situ of the skin in the TIL 100 group.
Compared to etanercept and placebo, both 100 mg and 200 mg doses of tildrakizumab demonstrated low rates of malignancies over time.
CONCLUSION
Efficacy trials of tildrakizumab following 52 weeks use show PASI ≥75 was maintained in >85% of those who initially responded to tildrakizumab 100 mg or 200 mg by 16 weeks.2 Here, low incidence rates of MACE, severe infections, and malignancies were observed in individuals with moderate-to-severe plaque psoriasis administered tildrakizumab for up to 148 weeks, comparable to rates for individuals treated with either etanercept or placebo. As such, the long-term safety profile of tildrakizumab is favourable.







