Presenters: Christopher E.M. Griffiths,1 Kenneth Gordon,2 Wolf-Henning Boehncke,3 Steven Feldman4
- Dermatology Centre, Salford Royal Hospital, University of Manchester, Manchester, UK
- Medical College of Wisconsin, Milwaukee, Wisconsin, USA
- Division of Dermatology and Venereology, Geneva University Hospital, and Department of Pathology and Immunology, University of Geneva, Geneva, Switzerland
- Wake Forest School of Medicine, Winston-Salem, North Carolina, USA
Disclosure: Prof Griffiths has received honoraria and/or research grants from Abbvie, Almirall, Celgene, Galderma, Janssen, Leo Pharma, MSD, Novartis, Sandoz, and UCB Pharma. Prof Gordon has received research support and honoraria from AbbVie, Amgen, Boehringer Ingleheim, Celgene, Eli Lilly, Janssen, and Novartis and honoraria from Dermira and Sun Pharma. Prof Boehncke has received honoraria as a speaker and/or advisor from Abbvie, Almirall, Celgene, Janssen, Leo Pharma, Lilly, Novartis, and UCB. Prof Feldman has received research, speaking, and/or consulting support from Galderma, GSK/Stiefel, Almirall, Leo Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate, and National Psoriasis Foundation. Prof Feldman is the founder and majority owner of www.DrScore.com and a founder and part owner of Causa Research.
Acknowledgements: Writing assistance was provided by Paul Scutt, Ascend, Manchester, UK.
Support: The publication of this article was funded by Janssen via an education grant. The views and opinions expressed are those of the authors and not necessarily those of Janssen.
Citation: EMJ Dermatol. 2018;6[1]:79-87; DOI/10.33590/emjdermatol/10313833. https://doi.org/10.33590/emjdermatol/10313833.
Overview
Guselkumab is a monoclonal antibody targeting IL-23 that is approved for the treatment of patients with moderate-to-severe plaque psoriasis. Two of the posters reviewed in this article provide new insights into the clinical efficacy of guselkumab in patients with plaque psoriasis from the VOYAGE trials, firstly among those previously failing to respond to adalimumab and secondly in the setting of drug withdrawal and subsequent retreatment. In addition, data from a study reporting 56-week results from a Phase IIa study exploring the efficacy and safety of guselkumab in patients with psoriatic arthritis (PsA) are reviewed. The article concludes with a summary of the results of a survey highlighting the potential importance of evaluating gastrointestinal (GI) signs and symptoms during the management of patients with psoriasis.
Clinical Response After Guselkumab Treatment Among Adalimumab PASI 90 Non-Responders: Results from the VOYAGE 1 and 2 Trials (Poster P042)
Professor Christopher E.M. Griffiths
Guselkumab is a fully human monoclonal antibody that selectively binds to the p19 subunit of IL-23, thereby inhibiting interaction with the IL-23 receptor and preventing downstream release of proinflammatory mediators.1,2 Guselkumab is approved in the USA and Europe for the treatment of adults with moderate-to-severe plaque psoriasis.1,2 The pivotal clinical trial programme for guselkumab in patients with plaque psoriasis included two Phase III, double-blind, placebo and adalimumab- controlled studies, VOYAGE 1 and 2.3,4 The analysis presented in this article was conducted to evaluate clinical response and patient- reported outcomes among those patients who initially received adalimumab and failed to achieve Psoriasis Area Severity Index (PASI) 90 responses in VOYAGE 1 and 2 and were subsequently switched to guselkumab. In addition, the safety of the crossover to guselkumab was explored.
As this analysis focussed on the patients in VOYAGE 1 and 2 who were initially randomised to adalimumab, those initially randomised to placebo or guselkumab are not discussed herein. In VOYAGE 1, 334 patients were initially randomised to adalimumab 80 mg subcutaneously at Week 0, followed by 40 mg at Week 1 and 40 mg every 2 weeks thereafter through to Week 47.3 All adalimumab-treated patients switched to guselkumab 100 mg at Week 52 and continued to receive guselkumab every 8 weeks until Week 100. The present analysis focussed on the 138 adalimumab-treated patients who were PASI 90 non-responders at Week 52. A similar initial adalimumab treatment regimen was used in VOYAGE 2 (n=248),4 with the exception that patients were switched to guselkumab 100 mg at Week 28. Patients subsequently received a second guselkumab dose at Week 32 and then every 8 weeks until Week 100. In VOYAGE 2, 112 adalimumab- treated patients were PASI 90 non-responders at Week 28 and were included in this analysis.
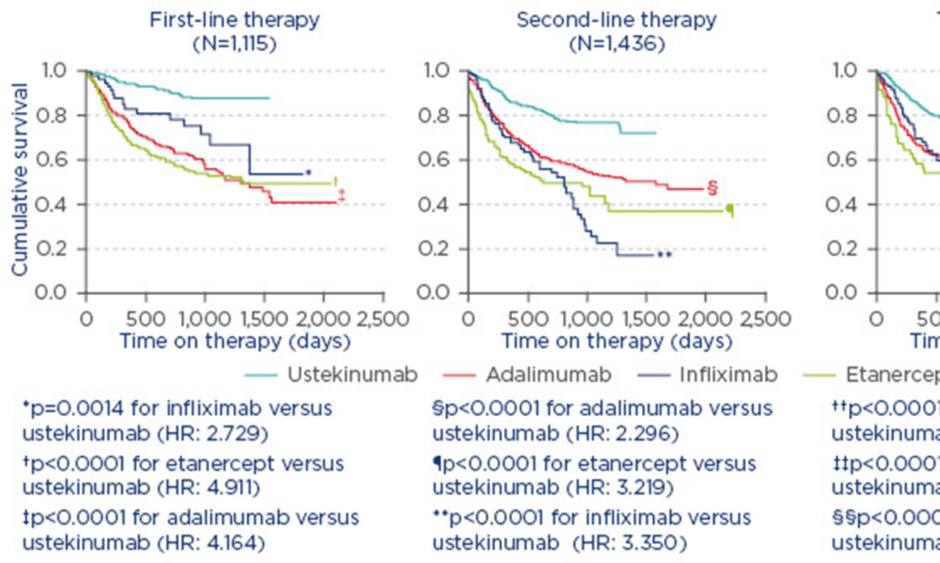
The results of the analysis revealed a robust clinical response associated with switching to guselkumab among adalimumab-treated patients who had initially failed to achieve PASI 90 at Week 52 and 28 in VOYAGE 1 and 2, respectively. At Week 100, after ~1 year of guselkumab treatment following adalimumab non-response, 73% and 42% of patients achieved a PASI 90 and 100 response, respectively, in the VOYAGE 1 trial (Figure 1A). Similarly, in VOYAGE 2, 75% and 43% of adalimumab non-responders had PASI 90 and 100 responses at Week 100, respectively, ~1.5 years after switching to guselkumab (Figure 1B).4 Improvements were also noted in the proportion of patients achieving an Investigator’s Global Assessment (IGA) score of 0 or 1 (cleared or minimal) after crossing over to guselkumab. By Week 100, 79% and 81% of adalimumab PASI 90 non-responders who switched to guselkumab had achieved IGA scores of 0 or 1 in VOYAGE 1 and 2, respectively.
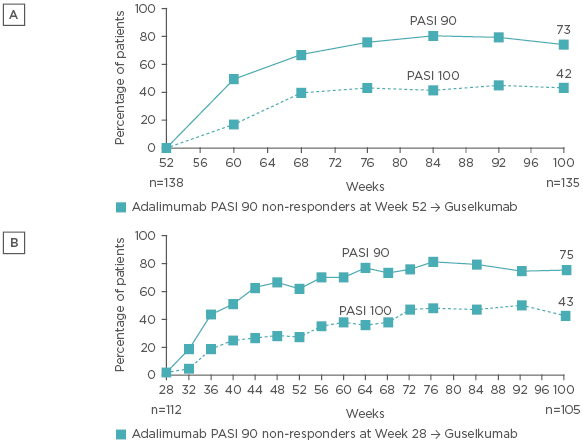
Figure 1: Proportion of PASI 90 and 100 responders among adalimumab PASI 90 non-responders who crossed over to guselkumab at Week 52 in VOYAGE 1 (A) and at Week 28 in VOYAGE 2 (B).
Analyses were performed using non-responder imputation through to Week 72 for Figure 1B and using observed data after applying treatment failure rules for Figure 1A and for Week 76–100 for Figure 1B.
PASI: Psoriasis Area Severity Index; →: crossover.
To further explore the impact of switching from adalimumab to guselkumab on patients, effects on health-related quality of life were analysed using the Dermatology Life Quality Index (DLQI) Score and patient-reported psoriasis symptoms and signs were assessed using the Psoriasis Signs and Symptoms Diary (PSSD). Adalimumab PASI 90 non-responders who switched to guselkumab achieved improvements in DLQI in both VOYAGE 1 and 2; the proportion of patients achieving DLQI scores of 0 or 1 increased from 25% to 75% from Week 48 to 100 in VOYAGE 1 and from 14% to 65% from Week 28 to 100 in VOYAGE 2. Improvements were also observed in the proportions of adalimumab PASI 90 non-responders achieving PSSD symptoms or signs scores of 0 following crossover to guselkumab. By Week 100 in VOYAGE 1, 33% of patients had achieved a PSSD symptom score of 0 and 19% had achieved a PSSD sign score of 0. In VOYAGE 2, 33% and 18% of patients achieved PSSD symptom and sign scores of 0, respectively.
No new safety signals were observed following crossover to guselkumab in adalimumab-treated patients, with the safety profile consistent with the overall guselkumab safety data previously reported from VOYAGE 1 and 2.5 Among the pooled population of patients in VOYAGE 1 and 2 (data through Week 100), rates of serious adverse events (AE) per 100 patient years in those treated with adalimumab (prior to guselkumab) and in those who crossed over to guselkumab were 7.77 and 4.44, respectively. Similarly, there was no notable elevation in the incidence of AE of interest with crossover to guselkumab: for adalimumab (prior to guselkumab) and adalimumab crossover to guselkumab groups, the incidence rates per 100 patient years were 1.8 and 0.0 for serious infections, respectively, 0.4 and 0.2 for major adverse cardiovascular events, respectively, 0.4 and 0.8 for non-melanoma skin cancer, respectively, and 0.4 in both groups for malignancy excluding non-melanoma skin cancer.
In summary, this analysis of data from the VOYAGE 1 and 2 studies established that, among adalimumab PASI 90 non-responders, switching to guselkumab provided robust levels of clinical response, enhanced health-related quality of life, and improved psoriasis signs and symptoms.
Long-Term Efficacy of Guselkumab Treatment After Drug Withdrawal and Retreatment in Patients with Moderate-to-Severe Plaque Psoriasis: Results from VOYAGE 2 (Poster P049)
Professor Kenneth Gordon
The VOYAGE 2 study4 was a Phase III, double-blind trial that investigated the efficacy and safety of guselkumab compared with adalimumab in patients with moderate-to- severe psoriasis. Following the initial 28-week active comparator period, the study design of VOYAGE 2 included a withdrawal and retreatment period that explored the comparative clinical efficacy and safety of continued guselkumab therapy versus withdrawal and retreatment upon relapse. Given that discontinuation of biologics, and in some instances retreatment, is a relatively common occurrence in patients with psoriasis,6 it is important to understand the impact of such events on clinical efficacy and safety. The study presented here reports the long-term results from the withdrawal and retreatment phase of VOYAGE 2.
In VOYAGE 2, 375 patients who were originally randomised to guselkumab 100 mg (at Week 0 and 4, and every 8 weeks thereafter) and achieved PASI 90 response at Week 28 were rerandomised to withdrawal (n=182) or continued guselkumab (n=193).4 Patients in the withdrawal group initially received placebo but were retreated with guselkumab upon loss of ≥50% of the PASI improvement achieved at Week 28; all patients who did not require retreatment were switched back to guselkumab at the Week 72 timepoint.
Patients who were randomised to receive continuous guselkumab therapy following a PASI 90 response at Week 28 typically maintained PASI 90 responses, with a PASI 90 response rate of 86% observed at Week 72 (Figure 2A). In contrast, PASI 90 response rates gradually declined in the group randomised to withdrawal following initial guselkumab PASI 90 response at Week 28, by Week 48, 37% of patients in the withdrawal group had PASI 90 response, and only 12% maintained PASI 90 response at Week 72. Among those patients in the withdrawal group who were retreated with guselkumab following loss of ≥50% of the PASI improvement achieved at Week 28, PASI 90 responses were recaptured in 88% of patients within 6 months of starting retreatment (Figure 2B).
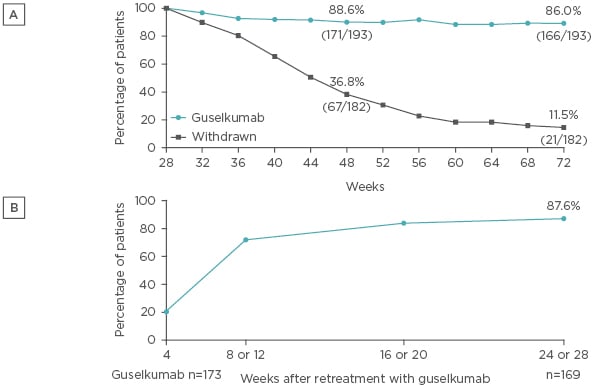
Figure 2: A) PASI 90 response among patients who were originally randomised to guselkumab, achieved a PASI 90 response at Week 28, and were subsequently randomised to withdrawal or continued guselkumab. B) Recapture of PASI 90 response following retreatment with guselkumab among patients randomised to withdrawal at Week 28.
Analysis performed with non-responder imputation for Figure 2A.
PASI: Psoriasis Area Severity Index.
The observed maintenance of PASI 90 response at Week 48 among approximately one-third of patients withdrawn from guselkumab in VOYAGE 2 has previously been reported to be associated with sustained suppression of serum cytokines, including IL-17A, IL-17F, and IL-22.7 Conversely, loss of response (PASI <75) is associated with increases in serum levels of these cytokines.7 For example, in those with loss of response, serum levels of IL-17A were significantly elevated from Week 28 levels (the time of withdrawal) at Week 40, 44, and 48 (p<0.01), and were significantly greater at Week 44 and 48 than the levels seen in those with maintained responses (p<0.05).
In addition to PASI response, the present study incorporated assessment of the effects of guselkumab withdrawal (and subsequent retreatment as needed) versus maintenance therapy on health-related quality of life using the DLQI score. Among those patients who had a PASI 90 response at Week 28, the proportion of patients achieving DLQI scores of 0 or 1 was maintained in the guselkumab maintenance group from Week 28 (70%) to Week 48 (69%) and increased to 80% by Week 100. In those who were randomised to withdrawal at Week 28, the proportion of patients with DLQI 0 or 1 scores decreased substantially after the switch to placebo, falling from 67% at Week 28 to 32% at Week 48. Retreatment with guselkumab in the withdrawal group led to recapture of the lost DLQI 0 or 1 response, with 68% of patients in the withdrawal group acheving DLQI 0 or 1 scores by the Week 100 timepoint. All withdrawal group patients reverted to guselkumab from Week 72 onwards.
In terms of safety and tolerability, no safety signals were observed with withdrawal and retreatment with guselkumab. The incidence of AE from Week 28–72 was similar in both the continued guselkumab and withdrawal groups, with 61% and 59% of patients per group experiencing ≥1 AE, respectively. Incidences of infections were similar in both maintenance and withdrawal groups (41% of patients in both) from Week 28–72, and there were no cases of tuberculosis, opportunistic infection, or serious hypersensitivity reactions. In those patients who were withdrawn from guselkumab, prior to retreatment with guselkumab there were two events of psoriasis rebound (≥125% increase in PASI score from baseline at any time during withdrawal) and no AE related to other forms of psoriasis.
In conclusion, the results of this long-term assessment of the efficacy of guselkumab treatment after withdrawal and retreatment following response at Week 28 provide several insights into guselkumab-based therapy. Firstly, the analysis demonstrated that continued treatment with guselkumab following PASI 90 response is associated with superior efficacy compared with treatment interruption, in terms of both maintenance of PASI 90 response over time and sustaining improvements in health-related quality of life. In contrast, guselkumab withdrawal leads to gradual declines in both of these variables. Maintenance of PASI 90 response after drug withdrawal was associated with continued suppression of IL-17A, IL-17F, and IL-22. Retreatment with 6 months’ guselkumab after withdrawal led to the recapture of PASI 90 response in the majority of patients, and there were no safety concerns identified among those initially withdrawn and subsequently retreated.
Efficacy and Safety Results of Guselkumab in Patients with Active Psoriatic Arthritis over 56 Weeks (Poster P119)
Professor Wolf-Henning Boehncke
Guselkumab is approved for the treatment of moderate-to-severe plaque psoriasis1,2 and is currently being evaluated in patients with PsA. PsA is a common comorbidity that has been estimated to affect approximately one in five patients with psoriasis,8 and significantly impairs patients’ physical function and ability to work.9 This poster describes the results from a randomised, double-blind, placebo-controlled, Phase IIa trial of guselkumab in PsA through Week 56.10
Eligible patients for this study included adults with active PsA, ≥3 tender and ≥3 swollen joint counts, and ≥3% body surface area affected by plaque psoriasis. In addition, patients were required to have previously experienced an inadequate response to current standard-of-care treatment, including non-biologic disease-modifying antirheumatic drugs, oral corticosteroids, or non-steroidal anti-inflammatory drugs. Prior exposure to an anti-TNF agent was permitted but limited to 20% of the enrolled population. Eligible patients were randomised 2:1 to receive guselkumab 100 mg subcutaneously or placebo at Week 0, 4, and every 8 weeks thereafter, until Week 44. Patients were subsequently followed-up until Week 56. At Week 16, those patients who achieved <5% improvement from baseline in swollen and tender joint counts were able to switch to open-label ustekinumab. The placebo-controlled period ended at Week 24, at which point placebo-treated patients were switched to guselkumab therapy until Week 44.
In total, 149 patients were randomised, with 49 receiving placebo and 100 receiving guselkumab. Baseline demographics and American College of Rheumatology (ACR) component measures were generally similar between the two groups. Twenty-seven patients switched to ustekinumab at Week 16 (placebo group: n=17; guselkumab group: n=10). Among those initially randomised to guselkumab, 84 patients completed the 56-week study. Twenty-nine patients randomised to placebo switched to guselkumab at Week 24, of whom 28 completed the remainder of the study.
The proportion of patients achieving a 20% improvement in ACR criteria (ACR 20) at Week 24 (the primary endpoint) was significantly greater with guselkumab (58% of patients) compared with placebo (18.4%; p<0.001). Significantly greater ACR 20 response rates were observed with guselkumab versus placebo at the first assessment timepoint (Week 4; p<0.001) and were sustained throughout the 24-week placebo-controlled period (p<0.05 to p<0.001). Among the group continuing guselkumab therapy after Week 24, ACR 20 responses rates were maintained, with 61% of patients achieving ACR 20 at Week 56. In addition, guselkumab therapy was associated with significantly greater response rates than placebo in terms of ACR 50 (34% versus 10%, respectively; p=0.002) and ACR 70 (14% versus 2%, respectively; p=0.023 [post-hoc analysis]) at Week 24, with response rates maintained to Week 56.
Improvements in ACR criteria with guselkumab were complemented by reductions in the severity of psoriasis, with significantly greater PASI 75, 90, and 100 response rates with guselkumab versus placebo at Week 24 (all p<0.001). In addition, the proportions of patients with unresolved enthesitis or dactylitis were significantly reduced in the guselkumab group versus placebo at Week 24 (p<0.05). Patient-reported health-related quality of life measures were significantly improved with guselkumab relative to placebo at Week 24, including when assessed via the Health Assessment Questionnaire (HAQ-DI) and the 36-item Short Form Health Survey (SF-36) physical and mental component scores (all p<0.01). At Week 24, a significantly greater proportion of patients achieved minimal disease activity with guselkumab than placebo (23% versus 2%, respectively; p=0.001). PASI response rates, enthesitis and dactylitis resolution rates, health-related quality of life scores, and minimal disease activity rate were generally well maintained to the end of the study with continued guselkumab therapy.
Guselkumab was well-tolerated over the 56-week study, with no injection site reactions reported among the 750 guselkumab injections administered. Through Week 24, incidences of AE and infections were comparable between the guselkumab and placebo groups (AE: 36% and 33%, respectively; infections: 16% and 20%, respectively). Longer guselkumab exposure through Week 56 did not lead to a disproportionate increase in the incidence of AE or infections. Serious AE were reported by six patients (6.0%) through Week 56 in the guselkumab group and two patients (2.0%) discontinued due to AE (leukopenia/neutropenia and pneumonia, respectively). A single malignancy (basal cell carcinoma) was reported by one patient (0.8%) who received guselkumab. Neutropenia was reported in four guselkumab-treated patients through Week 24 (three cases of Common Terminology Criteria for Adverse Events [CTCAE] Grade 2, which resolved spontaneously, and one case of CTCAE Grade 3, in whom guselkumab was discontinued and neutropenia resolved without treatment). There were no infections reported in the patients developing neutropenia, and no additional cases with Grade ≥2 occurred after Week 24. Increases in alanine transaminase/aspartate transaminase were generally comparable between guselkumab and placebo groups. There were no deaths, opportunistic infections, cases of active tuberculosis, or anaphylactic reactions.
In summary, the study demonstrated significant improvements in joint symptoms, physical function, psoriasis, enthesitis, dactylitis, and quality of life with guselkumab in patients with active PsA, with efficacy well-maintained through Week 56. Furthermore, guselkumab was well tolerated over the course of approximately 1 year of exposure.
Gastrointestinal Symptoms are Common in U.S. Patients with Moderate-to-Severe Psoriasis (Poster P112)
Professor Steven Feldman
Patients with plaque psoriasis are at increased risk of developing inflammatory bowel disease (IBD), with the risk increasing with higher degrees of psoriasis severity.11 Such concordance in disease incidence may arise from shared genetic susceptibilities and common inflammatory pathogenic pathways.12 Understanding the frequency of GI symptoms in patients with psoriasis is important, as the presence of GI disease could impact which treatments are chosen. This survey study was conducted to evaluate the prevalence of GI signs and symptoms among patients with plaque psoriasis.
An electronic survey was undertaken in the USA using an online opt-in patient panel/database, with data collected from January 2017 to February 2017. Patients with self-reported moderate-to-severe plaque psoriasis and healthy controls were eligible for inclusion in the survey, with psoriasis patients categorised into two subgroups: those with recent (within 4 months) exposure to biologic therapy (the PsORT group) and those without such exposure (the PsO group). Patients were evaluated for GI signs and symptoms consistent with IBD, and the frequency and severity of such symptoms were compared across groups. Patients with a diagnosis of IBD, irritable bowel syndrome, or other GI disorders with symptoms overlapping with IBD were excluded from the analysis. To further explore the impact of psoriasis on IBD risk, CalproQuest scores were calculated; CalproQuest scores have recently been proposed as a potential tool for identifying patients who have elevated faecal calprotectin levels and increased risk of IBD.13 The CalproQuest score is calculated from an IBD symptom questionnaire consisting of eight criteria (e.g., ‘Does the patient report a bloody stool?’), with results considered positive if ≥2 major criteria, or one major and two minor criteria, are met.13
In total, 915 patients with self-reported moderate-to-severe plaque psoriasis (450 of whom had recent biologic exposure) were enrolled in the survey, along with 1,411 healthy controls. Demographics were broadly comparable between groups, although patients in the plaque psoriasis cohort were on average younger than those in the healthy control group. Among those with psoriasis, almost all patients had a disease duration >1 year, and 39% and 21% reported having had psoriasis for >10 years in the PsO and PsORT groups, respectively. Substantially more patients in the PsORT group (35%) had been hospitalised within the last year for psoriasis versus the PsO group (3% of patients).
GI signs and symptoms were more common among those in the PsO and PsORT groups compared with healthy controls for all variables assessed, including stomach pain, feeling full or bloated, diarrhoea, mucus in the stool, and blood in the stool (Table 1). A significantly lower incidence of stomach pain, a full or bloated sensation, and diarrhoea were reported in those without versus with recent exposure to biologic. Incidences of mucus or blood in the stool were numerically, but not significantly, lower among PsO versus PsORT patients.
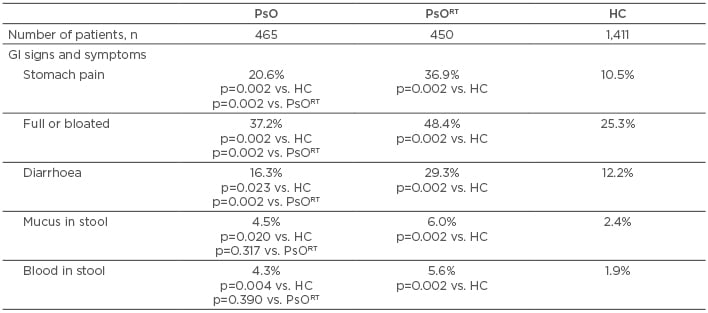
Table 1: Gastrointestinal signs and symptoms among patients with psoriasis and healthy controls.
*Within last 4 months.
GI: gastrointestinal; HC: healthy controls; PsO: psoriasis patients without recent biologic exposure; PsORT: psoriasis patients with recent biologic exposure.
Calculation of CalproQuest scores indicated a significantly greater proportion of patients with a positive CalproQuest result among the PsO group (10%) versus the healthy controls (6%; p=0.005). The greatest incidence of positive CalproQuest scores occurred among the PsORT group, with positive results for one in five patients (20%; p=0.002 versus both the PsO and healthy volunteer groups).
Although the cause of higher frequency of GI symptoms in those with recent biologic exposure was not assessed, one possible explanation is that patients with recent biologic exposure may have worse psoriasis, and that worse psoriasis has a greater association with IBD. Another possibility is that some agents used to treat moderate-to-severe psoriasis are associated with increased risk of IBD (in particular, IL-17 blockers) or simply GI intolerance (apremilast).
In summary, the present survey highlights that GI signs and symptoms are common in patients with moderate-to-severe plaque psoriasis, and occur at a higher incidence than seen in healthy controls. As such, it is important for healthcare professionals involved in the care of patients with psoriasis to consider assessing and monitoring GI signs and symptoms to identify patients who may be at risk of developing IBD.