Presenters: David Pariser,1 Stephen Tyring,2 Kristian Reich,3,4 Laura Ferris5
- Pariser Dermatology Specialists/Virginia Clinical Research, Inc., Eastern Virginia Medical School, Norfolk, Virginia, USA
- Center for Clinical Studies, Webster, Texas, USA
- Dermatologikum Berlin, Berlin, Germany
- SCIderm GmbH, Hamburg, Germany
- Department of Dermatology and Clinical and Translational Science Institute, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA
Disclosure: The authors have declared no conflicts of interest.
Acknowledgements: Writing assistance was provided by Michael Barker, Syneos Health, London, UK.
Support: The publication of this article was funded by Janssen via an education grant. The views and opinions expressed are those of the authors and not necessarily those of Janssen.
Citation: EMJ Dermatol. 2018;6[1]:88-93; DOI/10.33590/emjdermatol/10314012. https://doi.org/10.33590/emjdermatol/10314012
Overview
Plaque psoriasis is an autoimmune condition characterised by the development of red, dry, scaly skin lesions that cause irritation and pain for patients. It is a disabling and disfiguring condition and, alongside the physical effects, is associated with psychological comorbidities, including anxiety and depression.1 Combined effects of the condition are known to affect productivity at work, with increased rates of absenteeism.
Novel targeted therapies have the potential to transform treatment in this field. Adalimumab is a monoclonal antibody that inhibits TNF and has been approved in Europe since 2007 for the treatment of patients with moderate-to-severe chronic plaque psoriasis who are eligible for systemic therapy or phototherapy. Guselkumab is a novel IL-23-blocking monoclonal antibody that has been approved for use in the same indication as adalimumab in Europe since 2017. Personalised treatment is becoming more common and the delivery of therapeutics is a changing landscape, with a shift towards patients administering their own medication through novel devices.
This article reviews four posters displayed at the European Academy of Dermatology and Venereology (EADV) Congress 2018 that present results demonstrating the efficacy of guselkumab compared to adalimumab for the treatment of psoriasis, as measured by a range of outcomes, a favourable drug delivery system, and a higher drug survival rate overall.
Drug Survival is Superior Among Patients Treated with Guselkumab Compared to Adalimumab in the VOYAGE 1 Trial (Poster P1937)
Doctor David Pariser
Drug survival, defined as the probability that a patient will remain on a given therapy, is an important measure of the success of a therapeutic, especially in chronic conditions. Drug survival demonstrates the long-term tolerability and efficacy of an agent indicated in a condition and can show favourability over other therapies in head-to-head trials to measure treatment sustainability.
A post-hoc analysis of data collected in the VOYAGE 1 study was carried out to determine drug survival of guselkumab compared with the active comparator adalimumab.2 In VOYAGE 1, patients were randomised 1:1 to guselkumab (n=329) or adalimumab (n=334). Baseline demographic characteristics were comparable between the groups. Primary analyses of discontinuation for any reason up to 48 weeks of treatment were performed. Specific reasons for discontinuation were tabulated and a comparison of demographic and disease characteristics of patients discontinuing each treatment was carried out. Kaplan–Meier plots were produced to compare drug survival of guselkumab and adalimumab. The hazard ratio for risk of discontinuation of guselkumab versus adalimumab was calculated using Cox modelling. Secondary analyses were carried out, including evaluation of worsening disease or lack of treatment efficacy and adverse events.
Primary analyses compared baseline demographic characteristics of patients discontinuing the study drug. In the adalimumab group, patients discontinuing treatment had a higher median baseline body weight than those in the guselkumab arm (97.7 kg versus 84.9 kg, respectively). Other demographic and disease characteristics were comparable between discontinuing patients in both groups. Higher body weight has been associated with lower efficacy for a number of biologic agents, and this association has been reported to be more pronounced for adalimumab compared with guselkumab.2 This may be reflective of differences in immunogenicity or other factors affecting the serum levels of each drug and, therefore, its biologic availability and efficacy.
Guselkumab showed a superior drug survival rate compared with adalimumab at 48 weeks of treatment. Fifty-two (15.6%) patients in the adalimumab group discontinued the agent for any reason, compared with 28 (8.5%) patients in the guselkumab group. This difference in failure rate was statistically significant (p=0.0053) and the hazard ratio of 1.88 for discontinuing adalimumab versus guselkumab (95% confidence interval [CI]: 1.19–2.98; p=0.0070) was also statistically significant. It was suggested that the greater efficacy seen with guselkumab largely accounted for its superior drug survival compared with adalimumab.
Secondary analyses revealed that lack of efficacy or worsening of psoriasis was the most frequent reason for cessation of adalimumab, with 17 (5.1%) patients discontinuing as a result, compared to 3 (0.9%) patients in the guselkumab group (hazard ratio: 5.714 [95% CI: 1.675–19.500; Cox model p=0.0054]). For patients discontinuing treatment for reasons other than lack of efficacy or worsening psoriasis, drug survival was similar in the two groups; guselkumab had a survival rate of 97.0% compared to 98.2% with adalimumab (p=0.2790).
Overall, drug survival was superior for the guselkumab group compared with the adalimumab group at Week 48 in the VOYAGE 1 study. Drug survival can be assessed using data from clinical trials with an active comparator arm, as is the case in this analysis, but it should be noted that analysis of real-world data from drug registries in the post-approval setting is required to confirm these conclusions.
Association Between Improvements in Patient-Reported Outcomes and Absolute Psoriasis Area Severity Index Score: Results from VOYAGE 2 (Poster P1944)
Professor Stephen Tyring
VOYAGE 2, a double-blind, placebo and active comparator-controlled study, investigated the association between changes in patient- reported outcomes (PRO) and Psoriasis Area Severity Index (PASI) scores in patients with moderate-to-severe plaque psoriasis.3 A number of PRO measures were used to assess health-related quality of life (HRQoL).
Patients (N=992) were randomised 2:1:1 to one of three treatment groups, receiving either 100 mg guselkumab via subcutaneous injection at Weeks 0, 4, 12, and 20 (n=496); placebo at Weeks 0, 4, and 12, followed by 100 mg guselkumab via subcutaneous injection at Weeks 16 and 20 (n=248); or adalimumab via subcutaneous injection, 80 mg at Week 0, 40 mg at Week 1, and then 40 mg every 2 weeks through to Week 23 (n=248). PRO measures were assessed using three questionnaires and results were stratified by five thresholds, defined according to absolute PASI score: 0, >0–<1, ≥1–≤3, >3–≤5, and >5.
The Dermatology Life Quality Index (DLQI) assesses HRQoL with 10 dermatologic disease-specific questions, producing a combined total score from 0–30. A score <1 indicates no impact of disease on a patient’s daily QoL. In VOYAGE 2, there was a statistically significant association between lower PASI scores and proportions of patients with a DLQI score of 0 or 1 at Weeks 16 and 24 (p<0.0001 for both timepoints).
The Hospital Anxiety and Depression Scale (HADS) has two subscales, one for anxiety and one for depression, each producing a score ranging from 0–32. A score <8 on each respective subscale indicates no anxiety or depression. Both anxiety and depression scores correlated with PASI score in VOYAGE 2. For example, associations between HADS anxiety score at both Week 16 (r=0.20) and Week 24 (r=0.16) were statistically significant (p<0.0001 for both). Similarly, a statistically significant correlation between HADS depression score and PASI score was found at both Week 16 (r=0.27) and Week 24 (r=0.22) (p=<0.0001 for both).
Finally, the Medical Outcomes Study 36-Item Short Form (SF-36) derives mental and physical component summary scores, ranging from 0–100, from eight multi-item scales. A score ≥50 is indicative of normal HRQoL. Mental component scores ≥50 were significantly correlated with lower PASI scores at both Week 16 (r=0.29) and Week 24 (r=0.25) (p<0.0001 for both). Scores ≥50 in the physical component also showed a relationship with PASI assessment at Week 16 (r=0.40) and Week 24 (r=0.30) (p<0.0001 for both).
Improvement in absolute PASI score was strongly associated with improvement in HRQoL in all PRO measures that were investigated, showing statistically significant correlations in every measure used.
Association of Absenteeism and Presenteeism with Anxiety and Depression in Patients with Moderate-to-Severe Psoriasis and Improvement After Treatment: Results from the VOYAGE 2 Trial (Poster P1921)
Doctor Kristian Reich
Analysis of the effect of psoriasis on productivity, absenteeism, and presenteeism was also carried out using data from the VOYAGE 2 study.4 Alongside physical manifestations of the condition, psoriasis is associated with psychological comorbidities and either or both can affect productivity, absenteeism, and presenteeism. The methodology of VOYAGE 2 up to Week 24 is described in the previous section. At Week 28, patients receiving guselkumab 100 mg subcutaneous injection at Weeks 0, 4, 12, and 20 who achieved ≥90% improvement in PASI were re-randomised to guselkumab 100 mg every 8 weeks or placebo. Responding patients who received placebo at Weeks 0, 4, and 12 and guselkumab 100 mg subcutaneous injection at Weeks 16 and 20 received placebo at Week 28; non-responders in this group continued guselkumab treatment. Patients who had been receiving treatment with adalimumab subcutaneous injections were given placebo at Week 28 if they had responded to treatment or crossed to guselkumab therapy. One hundred and ninety-three patients were randomised to guselkumab at Week 28. In all groups, patients received guselkumab upon loss of response on placebo.
Absenteeism and presenteeism data through to Week 48 of the study were presented. Absenteeism was reported using the DLQI question: ‘Over the last week, has your skin prevented you from working or studying? [Yes=3]. If No, how much has your skin been a problem at work or at school? [A lot=2, A little=1, Not at all=0].’ A score for presenteeism was derived from responses to the following domain from the Work Limitations Questionnaire: time management, physical demands, mental–interpersonal demands, and output demands. HADS responses were used to evaluate the impact of depression and anxiety on productivity.
At baseline in all treatment arms, 22.9% of study participants reported that their skin had prevented them from working or studying, according to their response to the DLQI question; patients who had anxiety or depression at baseline were more likely to report this outcome (43.2%) than those who did not (17.1%). Patients in active employment had HADS scores that correlated with productivity evaluation based on their responses to the Work Limitations Questionnaire domains (HADS anxiety: r=0.59; HADS depression: r=0.64; p<0.001 for both).
Guselkumab was shown to be an effective treatment in terms of work-related disease impact. At Week 24, 82% of patients treated with guselkumab who had scored 3 on the DLQI domain question at baseline now reported a score of 0, compared to 50% of patients treated with adalimumab (p<0.001). With further follow-up to Week 48, 83% of guselkumab patients had reduced their DLQI score from 3 at baseline to 0. Patients who were randomised to guselkumab treatment at Week 28 showed an improvement in absenteeism and presenteeism up to Week 48.
The improvement in presenteeism at Week 24 was significantly greater in the guselkumab group compared to the adalimumab group, in three out of the four domains. The mean percentage improvements for guselkumab and adalimumab, respectively, were 38% versus 21% in physical demands, 42% versus 22% in mental–interpersonal demands, and 40% versus 16% in output demands. A sustained improvement in presenteeism was seen at longer-term follow-up at Week 48. Mean improvements from baseline were 46% in physical demands, 37% in time management, 49% in mental–interpersonal demands, and 49% in output demands.
Guselkumab demonstrated an advantage over adalimumab in patients both with and without anxiety and depression when measured by the DLQI domain absenteeism question. In patients treated with guselkumab, 73.5% of study participants with anxiety or depression who scored 3 on the DLQI assessment at baseline reported a score of 0 at Week 24, compared to 38.7% of patients treated with adalimumab (p=0.002). For patients without depression or anxiety, 88.9% of patients scoring 3 in the DLQI assessment at baseline had improved to a score of 0 at Week 24 when treated with guselkumab, compared to 64.0% of patients treated with adalimumab (p=0.006). The odds ratio for patients treated with guselkumab achieving a score of 0 on the DLQI assessment at Week 24 was 2.85 (95% CI: 1.83–4.46) compared to patients receiving adalimumab (p<0.0001).
In conclusion, anxiety and depression have significant impacts on productivity at work, affecting absenteeism rates, productivity, and presenteeism in patients with moderate-to-severe psoriasis. Treatment with guselkumab demonstrated significantly better outcomes for patients in absenteeism and presenteeism domains compared to treatment with adalimumab.
Evaluation of the Usability and Acceptability of a Novel, Patient-Controlled Injection Device for the Treatment of Moderate-to-Severe Psoriasis: Results from the Phase III ORION Study (Poster P1898)
Doctor Laura Ferris
The Phase III ORION study is a multicentre, randomised, double-blind, placebo-controlled study of guselkumab in patients with moderate-to-severe psoriasis. At baseline, 78 patients were randomised to placebo (n=16) or guselkumab (n=62).5 All study agents were administered using a manually-operated, patient-controlled, disposable device that delivered the contents of a pre-filled syringe via subcutaneous injection. The device included an automatically locking safety guard to shield the needle and prevent accidental needle stick injury. This poster presented results of patient-reported satisfaction with the self-injection device, including its ease of use and their experience of psoriasis after initiating treatment delivered in this way, along with assessment of correct use of the device by an objective observer.
Objective usability of the device was assessed at Week 0 through a three-step Observer Injection Checklist that reported on the patients’ removal of the device cap, positioning of the device, and completion of the injection. Patient-rated acceptability was assessed post-injection at Weeks 0, 4, and 12 using a Self-Injection Assessment Questionnaire (SIAQ) consisting of six domains (feeling about self-injections, self-image, self-confidence, pain and skin reactions during or after injections, ease of use of the injection device, and satisfaction with self-injection) (Table 1). The domains ‘feeling about self-injection,’ ‘self-confidence,’ and ‘satisfaction with self-injection’ were also scored pre-injection at Week 0. The SIAQ used a semantic Likert-type scoring method and responses were transformed into scores of 0–10 (worst to best). A three-question patient rating system was also used to assess speed of injection, handle design of the device, and ease of identifying completion of the injection.
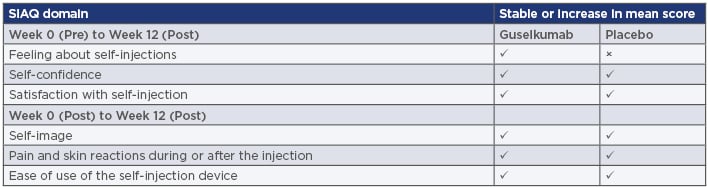
Table 1: Summary of score changes in six patient-reported Self-Injection Assessment Questionnaire domains measured in the ORION Study.
SIAQ: Self-Injection Assessment Questionnaire.
Adapted from Ferris et al.5
Patients in both groups were primarily successful in the Observer Injection Checklist assessment for device-related problems associated with the injection at Week 0, with 98.7% (77 out of 78) of patients observed to have successful, problem-free injections. One patient in the guselkumab group used the device improperly. This indicates favourable usability, as assessed objectively.
Scores for the three SIAQ domains assessed prior to the first injection, ‘feeling about self-injection,’ ‘self-confidence,’ and ‘satisfaction with self-injection,’ ranged from 6.59–8.23 and showed a tendency to remain high or increase at assessment post-injection at Week 0 and at Week 12. In the self-confidence domain, mean SIAQ score in the placebo group was 6.35 at Week 0 pre-injection, increasing to 8.21 at Week 12. Patients treated with guselkumab had mean scores of 6.67 at Week 0 pre-injection and 8.48 at Week 12. This indicated an increase in self-confidence over time when using the patient-controlled injection device.
Similarly, SIAQ scores for ‘satisfaction with self-injection’ increased from pre-injection at Week 0 to Week 12. In the placebo group, the mean score at pre-injection was 6.33, increasing to 9.26 at Week 12, compared to 6.65 and 9.64, respectively, for patients treated with guselkumab. Mean SIAQ scores for ‘feeling about self-injection’ decreased from 8.18 at pre-injection (Week 0) to 7.50 at Week 12 in the placebo group and increased slightly from 8.23 to 8.45 in the guselkumab group. Additionally, SIAQ scores only measured post-injection (at Weeks 0, 4, and 23) were favourable across all treatment domains and at all timepoints, suggesting that the patient-controlled delivery device was well-accepted by study participants. Median self-image scores remained at 10 from Week 0 to Week 12 in both placebo and guselkumab groups.
SIAQ reports of pain and skin reactions during or after the injection were relatively uncommon. A median score of 10, indicating no pain or skin reaction at all, was reported at all timepoints throughout the study. Mean scores also remained stable; in the placebo group, the mean score was 9.86 at Week 0, 9.77 at Week 4, and 9.89 at Week 12. In the guselkumab group, these were 9.82, 9.75, and 9.83, respectively, indicating that the injection device was well tolerated by users operating it correctly. SIAQ scores for the ease of use of the self-injection device remained consistent at the three timepoints measured in both groups. For the total study population (N=78), the mean ease of use was 8.81 at Week 0, 9.19 at Week 4, and 9.24 at Week 12.
Following the first injection at Week 0, study participants from across the treatment groups said that the injection device was easy or very easy to use; 94.9% of patients were either satisfied or very satisfied with the current method of medication administration. Results from the three-question patient questionnaire indicated that the injection device was well tolerated and well received by patients. Across both treatment groups (n=75), 97.3% of study participants either agreed or strongly agreed with the statements ‘I liked being able to inject the medication at a speed that was comfortable for me’ and ‘The design of the handle made the device easy to use’; furthermore, 94.7% of patients agreed or strongly agreed that they were able to easily tell when the injection was finished.
Although this study did not compare the use of the self-injection device to other drug delivery systems, the results confirmed that the patient-controlled device was well tolerated and accepted by study participants, who had a favourable experience when using it, and showed an association between using the device and successful, problem-free injections.
Conclusion
Guselkumab has been assessed in the post-approval setting for the treatment of plaque psoriasis and a number of reporting measures, including safety and efficacy, usability, and PRO, have been used to determine its suitability. Guselkumab has been evaluated against adalimumab as a treatment for plaque psoriasis in active comparator studies, with generally favourable outcomes.






