Presenters: Ellen Kim,1 Elaine S. Gilmore,2 Brian Poligone,2,3 Christiane Querfeld4
1. Department of Dermatology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA
2. Rochester Skin Lymphoma Medical Group, PLLC, Fairport, New York, USA
3. Rochester General Hospital Research Institute, Rochester, New York, USA
4. City of Hope Comprehensive Cancer Center and Beckman Research Institute, Duarte, California, USA
Disclosure: Prof Kim has received research funding or consultant fees from Actelion, Galderma, MedImmune, and Soligenix; and serves on the Scientific Advisory Board for Helsinn Therapeutics. Dr Gilmore has received research and consultancy fees from Helsinn Therapeutics. Dr Poligone has received research funding or consultant fees from Astex, Bioniz Therapeutics, Helsinn Therapeutics, Innate Pharma, Kyowa Kirin, MiRagen Therapeutics, Soligenix, Regeneron, and Stemline Therapeutics; and honoraria from Kyowa Kirin, Mallinckrodt, Regeneron, and Stemline. Dr Querfeld has served as a steering committee member, advisory board member, speaker, consultant, or received research funding from Helsinn/Actelion, Celgene, Trillium Therapeutics, miRagen Therapeutics, Bioniz Therapeutics, Kyowa Kirin, Medivir, and Mallinckrodt.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The publication of this article was funded by Recordati Rare Diseases.
Meeting Summary
The 4th World Congress of Cutaneous Lymphoma (WCCL) included several presentations reporting results from real-world studies of chlormethine use. Also known as mechlorethamine or nitrogen mustard, chlormethine is a topical therapy approved for mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL).1,2 It is an alkylating agent with mechanisms of action including cross-linking of DNA in rapidly dividing cells, leading to cell death.1 A high level of efficacy of chlormethine has been shown in MF-CTCL,3,4 yet topical use has been limited by cutaneous intolerance, with early discontinuations being a common occurrence. In 2013, the evaluation of a gel formulation of chlormethine/mechlorethamine (CL Gel) demonstrated noninferiority to pharmacy-compounded ointment in terms of response rates for the primary endpoint of Composite Assessment of Index Lesion Severity (CAILS).3,5 This resulted in the approval of topical chlormethine/mechlorethamine 0.016% w/w gel (equivalent to 0.02% chlormethine HCl) in the USA in 2013 and in Israel6 and Europe in 2016.7
In this article, data are summarised from the PROVe study presented by Prof Kim regarding real-world experience of CL Gel use in individuals with MF-CTCL in the USA. A lower percentage of participants experienced dermatitis than previously reported,3 which was likely because of the reduced dosing frequencies used by many in the study compared with product label recommendations, in addition to concomitant topical steroid use by most participants. Next, preliminary data are reported from the ongoing MIDAS study investigating contact dermatitis associated with CL Gel, presented by Dr Gilmore and Dr Poligone. These data suggest that people with MF-CTCL who develop dermatitis may possess a generalised allergic phenotype. Finally, a summary of Dr Querfeld’s presentation of results from a USA study that revealed an association between number of patients treated (physician experience) and treatment duration for clinicians prescribing CL Gel to patients affected by MF-CTCL.
The PROVe Study: Real-World Experience with Chlormethine Gel and Other Therapies in the Treatment of Patients with Mycosis Fungoides-Type Cutaneous T-Cell Lymphoma
Professor Ellen Kim
An enhanced understanding of real-world CL Gel usage could help improve the management of patients with MF-CTCL. The objectives of the PROVe study were to describe and assess treatment patterns, efficacy, safety, and health-related quality of life outcomes (HR-QoL) in patients with MF-CTCL treated with CL Gel and other therapies in a real-world setting.8 This prospective, open-label, single-arm, observational study enrolled 298 adults (mean age: 61.7 years; male: 60.1%; Stage IA/IB: 60.4%) in 46 centres across the USA who were actively using topical CL Gel during standard-of-care visits. Approximately 78% of participants were receiving concomitant skin-directed therapy and 30% were receiving concomitant systemic therapy. Clinical response was defined as ≥50% reduction from baseline in body surface area involvement at 12 months. Skin disease-specific HR-QoL was assessed by the Skindex-29 questionnaire score.9 Patients were followed-up for 2 years. The study used ‘by-time’ statistical analysis to examine the different response patterns over time during CL Gel treatment.
At 12 months, 45.1% of those with Stage IA–IB MF-CTCL (n=180) showed a clinical response to treatment, with the peak response occurring at 18 months. Approximately 37% of these patients who responded showed a clinical response at 1 month and 67% at 18 months. Compared to a pivotal randomised controlled trial of CL Gel in 2013,3 peak clinical response was higher (66.7% versus 55.7%, respectively) in the PROVe study, but occurred later. This is likely because of the flexibility in dosing with more gradual dose escalation which was permitted in the PROVe study.
Post hoc by-time analysis was used to highlight changing response rates over time (all MF-CTCL stages), in which considerable variation in treatment response was observed. Early responders typically showed a clinical response at 1–4 months of treatment, whereas some late responders did not show a clinical response until 17 months following treatment initiation. Over the 2 years of the study, responders reported significantly better HR-QoL than nonresponders. The weighted mean Skindex-29 scores for impairment of emotions, symptoms, and functioning, for which higher scores indicate lower HR-QoL or higher impact of disease, were 26.6 versus 36.2, 25.3 versus 34.4, and 13.3 versus 21.2, respectively (p<0.001). Knowledge of these factors can help patients with MF-CTCL, and healthcare practitioners set expectations regarding typical response timelines.
As of February 2019, approximately half of the participants (44.6%) had experienced ≥1 CL Gel-related adverse event (AE), of which 93.0% were “skin/subcutaneous tissue disorders” at the application site.10 AE included mild-to-moderate dermatitis (12.8%), pruritis (9.7%), and skin irritation (7.4%).10 A serious AE occurred in 8% of patients, but none were CL Gel-related.10 Overall, a lower rate of skin-related AE was observed during the PROVe study compared with the pivotal 2013 study,3 which is likely because of concomitant topical corticosteroid use (the majority of participants had been receiving treatment for >30 days prior to the trial) and flexibility in the dosing schedule within the PROVe study.
Based on the dosage used during the clinical development programme, CL Gel product labels recommend that the 0.016% w/w gel is applied once daily.1,2 However, treatment should always be suspended if skin ulceration, blistering, or moderate-to-severe dermatitis is observed.1,2 Treatment can be restarted at a reduced frequency of once every 3 days once symptoms improve, and then gradually increased.1,2 In the PROVe study, most participants (75%) applied CL Gel once daily, but a dose frequency change occurred during treatment in 63% of participants, and 29% experienced a dose interruption.10 This suggests that, for some participants, physicians decided to scale down the treatment to a lower frequency according to individual need or characteristics and indicates that continued treatment and close follow-up is important to maximise response potential.
The PROVe study is the largest prospective, observational study of real-world CL Gel use in the USA to date. In this setting, nearly one-third of participants with Stage IA-IB MF-CTCL showed a clinical response at Month 12, with two-thirds responding at Month 18. CL Gel was well tolerated with responders reporting significantly improved HR-QoL compared with nonresponders.
Incidence and Types of Contact Dermatitis, with Molecular Signature Patterns, After Chlormethine Gel Treatment in Patients with Mycosis Fungoides-Type Cutaneous T-Cell Lymphoma: The MIDAS Study
Doctor Elaine S. Gilmore and Doctor Brian Poligone
Cutaneous reactions at the site of CL Gel application can lead to noncompliance and treatment discontinuation.5 The MIDAS study was designed to investigate the incidence, form, and severity of cutaneous reactions, particularly contact dermatitis, following treatment with CL Gel in adults with Stage IA-IB MF-CTLC.11 MIDAS is an ongoing Phase II, nonrandomised, open-label, split-face, two-arm study. Over a 4-month period, participants apply the gel once nightly to an area ≥8 cm2 containing representative mycosis fungoides lesions; one-half of these lesions per patient are also treated once daily with triamcinolone 0.1% corticosteroid ointment. Participants who develop contact dermatitis are patch tested to characterise the reaction and identify causative agents, and biopsies are taken for pathohistological analysis and T-cell receptor (TCR) sequencing.
Contact dermatitis can be either a nonspecific skin response (irritant contact dermatitis) or a delayed hypersensitivity reaction to allergens (allergic contact dermatitis [ACD]). In the MIDAS study, these two forms of dermatitis were measured using the scoring atopic dermatitis (SCORAD)12 and scoring dermatitis (SCORD; a derivative of SCORAD currently under development) grading systems. Each system provides an overall score that increases with dermatitis severity.
Preliminary data from the MIDAS study indicate that roughly one-third of participants enrolled so far (n=26) developed severe contact dermatitis, with most of these cases being ACD. CAILS assessment showed similar clinical responses to CL Gel with or without concomitant corticosteroid treatment over 6 months. However, the average poorer SCORD score appeared to be much lower in the former group, suggesting that concomitant topical corticosteroid use may reduce the severity of dermatitis during CL Gel treatment.
Patch test results from participants who developed severe contact dermatitis revealed that the majority showed 2–3 positive reactions at 96 hours following application and that roughly two-thirds also reacted to numerous, seemingly unrelated allergens. Interestingly, histopathologic analysis has so far indicated that the skin reactions are characterised by a superficial and deep lymphocytic infiltrate with spongiosis and eosinophils, reminiscent of an insect or spider bite. Clinical responses have been observed in patients who developed either irritant contact dermatitis or ACD during treatment, despite previous unsuccessful topical steroid therapy.
As part of the MIDAS study, dermatitis lesion biopsies are being analysed by TCR sequencing to identify differentially abundant TCR clones, and repertoire similarity is being assessed using Morisita’s index. Preliminary data show that lesions contain novel clones, as well as an expanded population of pre-existing clones, but repertoire similarity in comparison to control biopsies (not exposed to CL Gel) varies between patients. In some participants, clinical response to CL Gel correlates with TCR molecular clonality.
In conclusion, preliminary results from the MIDAS study indicate that roughly one-third of people with MF-CTCL using CL Gel develop severe contact dermatitis, mostly ACD. Data suggest that those who develop ACD may possess an allergic phenotype that predisposes them to cutaneous reactions to common allergens. Contact dermatitis lesions that develop during treatment appear to show a clonal expansion of both pre-existing and novel TCR clones. Overall, patch testing appears to represent a useful tool for understanding the presentation of dermatitis in patients with MF-CTCL treated with CL Gel, which could help dermatologists improve management of this condition. The addition of topical corticosteroid to CL Gel treatment in this study did not impact efficacy but resulted in significantly less dermatitis. Future clinical trials are needed on larger patient cohorts to further the understanding of skin reactions to CL Gel, such as the REACH study by EORTC which plans to enrol 100 participants.13
Chlormethine Gel Treatment Duration as a Function of Clinician-Level Patient Volume for Mycosis Fungoides-Type Cutaneous T-Cell Lymphoma
Doctor Christiane Querfeld and Doctor Brian Poligone
Early discontinuation of CL Gel in people with MF-CTCL is often attributed to dermatitis, yet it is unclear whether other factors may also play a role. Dr Querfeld described the results of a recent USA study which aimed to evaluate the association between the number of patients prescribed CL Gel (a clinician’s “patient volume”) and both early discontinuation (<3 months of treatment) and overall treatment duration. USA dispensing-pharmacy records, representing the majority of USA utilisation of CL Gel, were analysed between 2013 and 2019. Close to 5,000 patients were assigned to over 2,000 clinicians. Almost all prescriptions were for a 1-month supply of CL Gel.
This study presented current, full, detailed, longitudinal data; however, it should be noted that treatment duration was defined by number of prescriptions dispensed rather than elapsed time, and neither line of therapy nor concomitant steroid use were captured.
Several important findings came out of this study:
- Most discontinuations occurred in the first 3 months. One-third of patients discontinued treatment during Months 1–3, after which the discontinuation rate fell by approximately 50%. Patients dispensed more than one course of medication were prescribed CL Gel for an average of 5 months.
- Individual clinicians varied considerably in terms of patient volume and treatment duration. A small number of clinicians (<3%) with >15 patients each were responsible for treating almost one-half of all patients. Most clinicians treated only one patient, with one-third treating between two and 15 patients.
- Clinicians with higher patient volume sustained longer treatment duration and, importantly, less early discontinuation (Figure 1). Clinicians with >15 patients dispensed CL Gel approximately six times per patient, while those with five to 15 patients dispensed it approximately four times. Patients were given just two prescriptions on average by clinicians with a single patient, with one-third of these clinicians only dispensing treatment once. As such, it was found that early discontinuation was significantly associated with lower volume of patients.
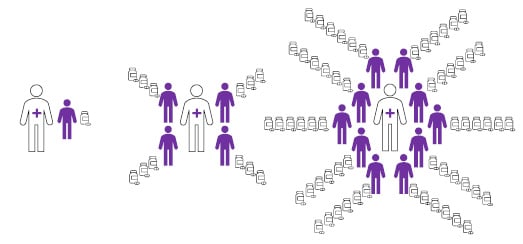
Figure 1: Infographic illustrating the association of patient volume with treatment duration (number of dispensed prescriptions).
Compared to clinicians with many patients with mycosis fungoides-type cutaneous T-cell lymphoma (right), those with fewer patients (left) were more likely to have patients that discontinued mechlorethamine at an early stage of treatment.
The longer treatment duration and lower rate of early discontinuation for patients treated by clinicians with high patient volumes may be attributable to the clinicians’ experience in managing patients with mycosis fungoides-type cutaneous T-cell and treatment-related adverse events such as dermatitis. Early discontinuation of CL Gel treatment may therefore represent a lack of physician experience regarding setting patient expectations and educating them on treatment adherence and toxicity.







