Speakers: Antony R. Young,1 Thierry Passeron2,3, Martin Josso4
1. St John’s Institute of Dermatology, King’s College London, London, UK
2. Department of Dermatology, University Hospital of Nice, Nice, France
3. INSERM U1065 Team 12, University Hospital of Nice, Nice, France
4. Suncare Development Laboratory, L’Oréal Research & Innovation, Paris, France
Disclosure: Prof Young has received an honorarium from La Roche-Posay and holds a consultancy with siHealth Ltd. Prof Passeron has received research grants and/or honoraria from L’Oréal, Bioderma, Beiersdorf, Galderma, ISIS Pharma, ISDIN, Pierre Fabre, SVR, and Symrise. Dr Josso is an employee of L’Oréal.
Acknowledgements: Medical writing assistance was provided by Drs Allison Kirsop and Penny Gray, Scientific Writers Ltd, Edinburgh, UK.
Support: The symposium and publication of this article were funded by La Roche-Posay Dermatological Laboratories International.
Citation: EMJ Dermatol. 2021;9[Suppl 2]:2-8.
Meeting Summary
Ultraviolet-A (UVA) radiation (320–400 nm) is the major component of terrestrial solar UV radiation (UVR). In contrast to UVB (280–320 nm), UVA is present all year round; is less impacted than UVB by latitude, season, and time of day; and has a greater penetration into the dermis. UVA has a wide range of deleterious effects on human skin at the cellular and molecular level, and long-wave UVA (UVA1 340–400 nm) has demonstrable roles in pigmentary disorders, skin ageing, skin cancers, and photodermatoses. Incorporating UVA protection into sunscreens is critically important for such clinical indications: both sun protection factor (SPF) and UVA protection factor (UVA-PF) must be considered when choosing sunscreens so that balanced photoprotection is offered in these conditions. UVA-related photoageing impacts dark and fair skin, and drug-induced phototoxicity can result from the interaction of topical or systemic agents with UVA. A good sunscreen combines potent and photostable UVR filters across a broad spectrum, comprising long-wave UVA and UVB in a vehicle that optimises efficacy, substantivity, and sensoriality, while being environmentally friendly. Safety of sunscreen is also important to assure its users of all skin types. Most users only rely on the SPF factor for choosing a sunscreen; however, between sunscreens with the same SPF, there is a high variability in the protection against UVA. In this symposium, Prof Young and Prof Passeron presented evidence on the biological and clinical impacts of UVA radiation on the skin and the associated clinical impact, and Dr Josso considered the composition of an ideal sunscreen, presenting evidence for the benefits of newer, technologically innovative sunscreens that offer well-balanced UVB and UVA protection alongside excellent tolerability, substantivity, and user acceptability.
Biological Impact of Ultraviolet-A Radiation
Terrestrial solar UVR consists of UVA (320–400 nm) and UVB (280–320 nm; typically around 295–320nm because of attenuation by the ozone layer), with UVB equalling approximately 5% of the total, despite being responsible for most of the damage caused to skin.1 However, the dose of exposure to UVA has been estimated to be approximately 17 times greater than UVB, irrespective of weather conditions or time of day.1,2 A growing body of evidence for UVA-induced damage to the skin over the past 20 years has led to an increasing focus on UVA protection in sunscreens.2 In 2006 (updated report 2018), the European Commission published a recommendation for sunscreen products to contain an SPF/UVA-PF ratio of ≤3,3 and in 2019, the U.S. Food and Drug Administration (FDA) advanced new proposals for broad-spectrum sunscreens to ensure safety and efficacy.3,4 Yet, concerns remain that a high proportion of sunscreens on the market may offer suboptimal UVA protection.5,6
Professor Antony R. Young
UVA constitutes around 95% of solar radiation.7 In contrast to UVB, it is much less dependent on the height of the sun and so is influenced to a lesser degree by latitude, season, and time of day.1 It is also poorly attenuated by the epidermis, and therefore has greater penetration into the dermis than UVB.
Prof Young outlined important biological effects on skin attributable to UVA at the cellular and molecular levels, underlying all clinical consequences of UVA exposure. UVA causes DNA damage to the epidermis, which is implicated in skin cancer.8,9 UVA causes oxidative damage to macromolecules via the generation of reactive oxygen species, erythema (though much less so than UVB),7 and pigmentation disorders, as discussed by Prof Passeron in this article. UVA also leads to changes in the skin microbiome, though the consequences of this are poorly understood.10 It also has a strong effect on epidermal gene expression9,11 and on the induction of matrix metalloproteinases (MMP), which characterise photoageing.12
Types of DNA damage by UVA include the formation of cyclobutane pyrimidine dimers (CPD), specifically thymine dimers, which occur preferentially in the basal layer of the epidermis and are known to be important in the development of skin cancers.12 Lawrence et al.9 have recently described the formation of dark CPD, which are delayed lesions that appear after in vivo exposure of human skin to radiation at the UVA/visible radiation boundary (385–405 nm). UVA also causes a diverse range of oxidative damage to DNA, notably 8-oxo-7,8-dihydro-2’-deoxyguanosine, which can be detected in urine after solar simulated radiation exposure in vivo.13 Interestingly, the number of CPD caused by long-wave UVA (UVA1, 340–400 nm) increases with epidermal depth, in contrast to attenuation seen with UVB (300 nm), demonstrating deeper damage with UVA.8 Moreover, UVA-induced dark CPD are stable between 1 and 24 hours postexposure, suggesting a lack of DNA repair.9 UVA also causes oxidative damage to enzymes that are essential for DNA nucleotide excision repair; a lack of these enzymes leads to an increase in the incidence of skin cancer in patients with xeroderma pigmentosum.7 Oxidation of lipids, particularly squalene, has also been observed in human skin in vivo.14
UVR-induction of MMP plays an important role in skin photoageing, as these enzymes degrade the structural proteins of the dermis.12 Different MMP are known to have different target molecules, with MMP1 and MMP12 targeting collagen and elastin, respectively.12 Solar UVR has been shown to induce MMP at the mRNA, protein, and activity levels, with different spectral regions preferentially inducing different targets.12 Selective staining for MMP1 (collagenase) and MMP12 (elastase) at 10 and 24 hours after one minimal erythema dose of UVA and UVB shows that UVA has a stronger effect than UVB on expression and activity of elastase at these times, while the opposite is true for collagenase.12 Preferential induction of elastase by UVA has implications for skin elasticity, as a result of elastin degradation, contributing to the effects of UVA on photoageing.
Clinical Impact of Ultraviolet-A on the Skin
Professor Thierry Passeron
Prof Passeron overviewed data on the clinical impact of UVA on human skin and highlighted the importance of using well-balanced sunscreen that protects adequately against UVA as well as UVB. UVA, especially long-wave UVA, accounts for 77‒80% of the UV solar spectrum and has a wide variety of deleterious effects on skin, including pigmentary disorders, skin ageing, skin cancers, and photodermatoses.13,14 Numerous studies have shown that well-balanced sunscreen (SPF/UVA-PF; persistent pigment darkening ≤3) shows superior efficacy against UV-induced pigmentation.15-17 While the backs of healthy volunteers exposed to a single dose of UV radiation with UV sunscreen protection of SPF50 UVA-PF13 demonstrated marked pigmentation, an increase in UVA protection to UVA-PF21 offered good protection against pigmentation. Similarly, over 7 days, combining SPF15 with UVA-PF15 versus UVA-PF3 showed a significant reduction in UV-induced pigmentation, demonstrating the importance of a better protection against UVA.15,16
Besides UVA, visible light (particularly high-energy visible [HEV] light, i.e., blue and violet light) also markedly affects pigmentation disorders, especially melasma, postinflammatory hyperpigmentation, and actinic lentigo;18-22 therefore, it is also important in protecting against HEV wavelengths. Long-wave UVA1 and HEV light have further been shown to have additive effects on pigmentation,23 suggesting that in clinical practice, prescriptions for patients with pigmentary disorders should protect against these two elements.
UVA, in addition to UVB, also contributes to skin ageing; daily exposure to UVA plays a key role in the skin ageing process because it constitutes the majority of UV light entering the dermis.24-27 In addition to fundamental evidence, there are several clinical cases supporting the impact of UVA radiation on the skin ageing process. Prof Passeron discussed two patient cases to clearly illustrate the effects of UVA on skin ageing. The first patient was a 69-year-old fair-skinned male with thickening and wrinkling of the skin restricted to the left side of his face. He classically demonstrated the impact of UVA: he had worked as a truck driver for 28 years and was chronically exposed to UVA on the left of his face, which, unlike UVB, is transmitted through glass. A similar effect is seen in dark-skinned individuals, for which marked differences can be seen between protected and exposed parts of the face.28 UVA and UVB are known to induce DNA damage29 and immunosuppression,30 promoting the development of melanoma;31 regular use of sunscreen decreases the risk of melanoma, as well as other skin cancers.32,33 A 24-month, prospective case–control study of immunocompromised organ transplant recipients showed that regular use of sunscreen SPF50 with high UVA-PF may help to prevent the development of further actinic keratoses and invasive squamous cell carcinoma,34 again indicating the need for balanced UVA/UVB protection for the prevention of skin cancers.
Robust data also exist on the association between UVA and photodermatoses,35-37 although different photodermatoses are associated with different wavelengths. Drug-induced phototoxicity results from the interaction of topical or systemic agents with UVA. Polymorphic light eruption may be induced by either UVA or UVB, and solar urticaria can be induced by UVA, UVB, and visible light wavelengths. Chronic actinic dermatitis is more often caused by UVB than UVA, while cutaneous porphyrias are activated by visible light in the 400–410 nm range. Finally, the action spectrum for both lupus erythematosus and dermatomyositis lies in both UVA and UVB ranges. Therefore, a balanced UVA/UVB protection is warranted for patients with photodermatoses.
Prof Passeron concluded his presentation with some key take-home messages: UVA has a demonstrated role in pigmentary disorders, skin ageing, skin cancers, and photodermatoses. Most patients choose a sunscreen on the basis of the SPF index, which reflects mostly UVB protection. The encircled UVA logo used on European sunscreen products indicates only that the SPF to UVA-PF ratio is ≤3 (i.e., has a UVA-PF of at least 20 for SPF50+), which is not sufficient for all the indications discussed in this article. Physicians need to consider SPF and UVA-PF38,39 and advise well-balanced photoprotection. For patients with pigmentary disorders, as well as individuals with solar urticaria and cutaneous porphyria, additional protection against visible light is also required.
Formulating the Optimal Sunscreens for Patients
Doctor Martin Josso
Following Prof Young and Prof Passeron, who discussed the importance of UVA protection, Dr Josso questioned what constitutes a good sunscreen, starting with a summary of the essential components.2 An optimal sunscreen should provide a powerful, photostable, broad-spectrum UV filtering system in a vehicle designed to optimise efficacy, substantivity, and sensoriality (the pleasant feel on the skin). The resulting formulation should be effective; environmentally friendly; and safe, an important factor to assure its compatibility for all skin types.
Sunscreens such as Anthelios Invisible Fluid (La Roche-Posay, Paris, France), which has a filtering system that combines six complementary UV filters and is photostable over the whole UV range, meet these criteria and offer broad-spectrum UV protection and strong protection against UVA. A poster presented by Dr Josso and colleagues at the EADV congress reported on a study assessing UVA protection of sunscreens with various formulations, tested using in vitro methods.6 This study found that while many products in the market exceed European recommendations for UVA protection levels, as many as one-third of products (none of them from the L’Oréal Group) were below this level. These results complement the findings of a survey of the in vivo UVA protection levels of leading SPF50+ dermocosmetic facial sunscreens on sale in Europe based on persistent pigment darkening assessments (M. Josso, data on file), which showed a wide range of UVA protection with surprisingly low-level UVA protection for two reference products on the market (Figure 1).
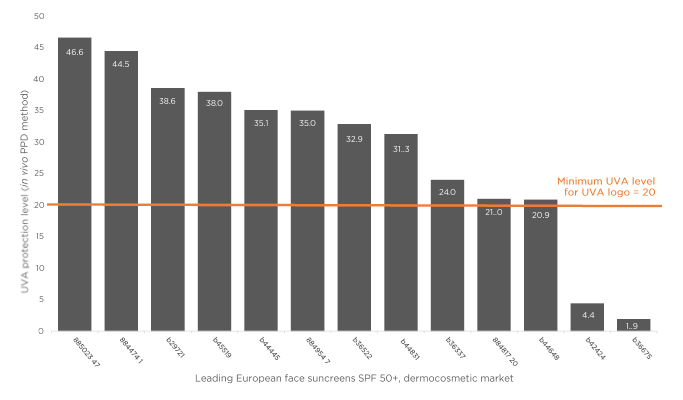
Figure 1: UVA protection level of leading dermocosmetic sunscreens: in vivo evaluation.
PPD: persistent pigment darkening; SPF: sun protection factor; UVA: ultraviolet-A.
M. Josso, data on file.
In addition to good retention and cosmetic features (UV filters can often impart a greasy, sticky feel), the formulation of UV sunscreen is critical to ensure compliance and thus optimal UV protection. Technological developments over the past decade, including the development of Netlock (L’Oréal, Paris, France) technology polymers to encapsulate the UV filters in jellified microdroplets, have provided significant improvements, allowing the UV filters to be spread evenly in a stable, fine distribution of droplets, with no oily skin feel, and without the need for surfactants.2 Netlock technology produces very fine and condensed droplets to ensure a more homogenous repartition, and the absence of surfactants allows such novel formulas to set rapidly on the skin, creating an invisible, flexible, and resistant film with no migration of the formula.
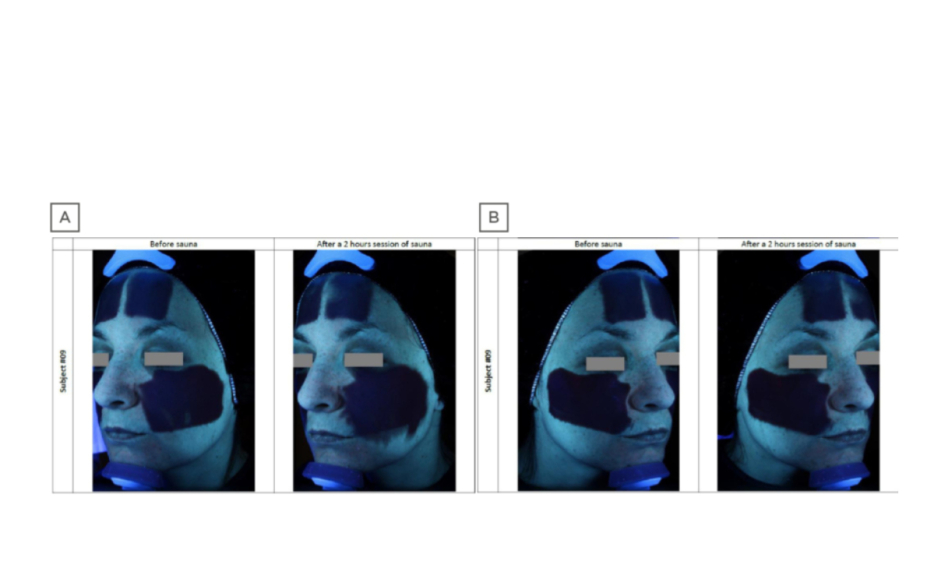
Stability and resistance to daily activities conferred by such novel formulations has been demonstrated;2 also, the difference in substantivity and stability was illustrated in a comparative study of a classical versus novel sunscreen formulation under conditions of extreme heat and humidity (2 hours in a sauna; Figure 2; Laboratoire Dermscan, data on file). The classical formula was shown to migrate on the skin, potentially impacting efficacy and causing eye stinging if applied close to the eyes. By contrast, Netlock technology formulation showed no migration. Regarding user acceptability and appreciation, the reduced stickiness and enhanced fluidity conferred by new technological developments in sunscreen formulation allow for easier application, while increased transparency avoids white marks, improving its appearance. The newer formulations are also rigorously tested to avoid any negative impact on coral and other aquatic life forms, and do not contain oxybenzone and octinoxate, which are now banned in some USA states.40
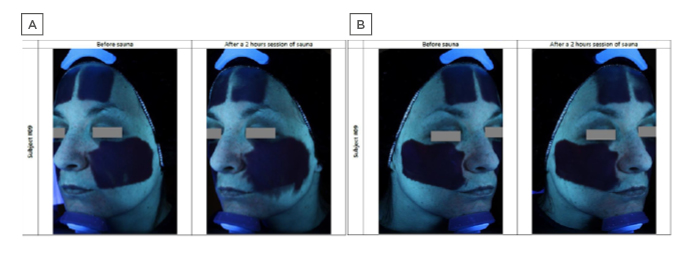
Figure 2: Comparative sunscreen stability under conditions of extreme heat and humidity, visualised with an ultraviolet camera.
A) Standard formula sunscreen showing significant migration after 2 hours’ sauna at 40 °C, 60% humidity. B) Novel sunscreen formula based on a semicrystalline polymer that encapsulates and stabilises the constituent ultraviolet-absorbing oil droplets, showing high resistance to migration under identical conditions.
A description of similar results showing stability after 3 hours or after daily work activities is provided in the literature.2
Laboratoire Dermscan, data on file.
Questions and Answers
Following the presentations, Prof Passeron chaired a question and answer session, during which the panel answered questions from delegates in the audience.
Q: What is the clinical impact of CPD in the basal layer?
Prof Young responded by saying that CPD located deeper in the epidermis were more damaging to the skin, particularly with UVA, because of the location of stem cells and melanocytes in the basal layer. Data from Prof Young’s research group (unpublished) have shown that repair in the basal layer with UVA is poorer than with UVB. This may be because of UVA-induced damage to the DNA repair enzymes, as discussed in his presentation.
Q: How important is UVA compared with UVB in the development of cancer?
Prof Passeron replied that both UVA and UVB are known to promote cancer. There is now good evidence from animal models, as well as clinical and epidemiological data, that UVA plays an important role in skin cancer and melanoma and stressed the importance of protection against both UVA and UVB.
Q: How can we determine that a sunscreen has balanced UVA/UVB protection?
Dr Josso responded that the only indicator of the UVA protection level in most sunscreen formulations in Europe is the encircled UVA logo, which shows that the UVA protection level is one-third of the UVB protection indicated by the SPF. Some reputable brands label UVA protection on their packaging, but this is quite rare. It is not easy for dermatologists to identify the level of UVA protection from the packaging. However, this could be improved if the European Commission mandated that sunscreen labelling should display the level of UVA protection.
Q: Which do you recommend more, physical or chemical sunscreen?
Prof Passeron responded that this is dependent upon the indication and the type of protection needed. For protection against long-wave UVA, a chemical sunscreen is clearly needed. However, with visible light, as in the case of pigmentary disorders such as melasma, physical protection is best, usually in the form of an iron oxide formulation; in this case, dermatologists need to combine chemical and physical sunscreen. It is now well-known which wavelengths impact different skin types and dermatoses; dermatologists should be aware of this and prescribe sunscreen accordingly.
Q: When it comes to daily SPF protection, do we need to prescribe SPF50+ or is SPF30 enough, and should it be prescribed all year round?
Prof Passeron responded that there was no consensus regarding daily photoprotection. It is dependent upon the skin type, geographical location, and other factors. Fair-skinned types need high SPF daily all year round. For individuals living in higher latitudes, it is nonessential to have high SPF; SPF30 would be sufficient in most instances. Since UVA is constant throughout the year, UVA protection is needed daily. Prof Young added that most people forget that SPF depends on sunscreen application thickness, applying approximately one-third of the sunscreen deemed necessary in a test situation. Therefore, they have much less protection. High SPF is a good idea if people are not using sunscreen properly, but if used in adequate quantities, SPF30 is likely to be high enough. Prof Passeron added that it was easy to direct patients to use the right amount of sunscreen. For melasma, for example, his clinic informs patients that the quantity used for the face is approximately equivalent to a teaspoonful.
Q: What is the most important radiation with regard to microbiome alteration?
Prof Young replied that he was not aware of any studies looking at the action spectrum for alteration of the skin microbiome. Moreover, it is an academic question as we are not only exposed to UVA or UVB, we are exposed to both. More research is needed to determine which wavelength is more important for microbiome alteration, and the associated consequences.