Presenters: Bruce Strober,1,2 Richard Warren,3,4 Kristian Reich5,6
1. Yale University, New Haven, Connecticut, USA
2. Central Connecticut Dermatology Research, Cromwell, Connecticut, USA
3. Dermatology Centre, Salford Royal NHS Foundation Trust, Salford, UK
4. Manchester NIHR Biomedical Research Centre, The University of Manchester, Manchester, UK
5. Center for Translational Research in Inflammatory Skin Diseases, Institute for Health Services Research in Dermatology and Nursing, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
6. Skinflammation® Center, Hamburg, Germany
Disclosure: Prof Strober has served as consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Equillium, GlaxoSmithKline, Janssen, LEO Pharma, Meiji Seika Pharma, Mindera, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi-Genzyme, Sun Pharma, and UCB Pharma; has been a speaker for AbbVie, Amgen, Eli Lilly, Janssen, and Ortho Dermatologics; has been a scientific director (consulting fee) for the Corrona Psoriasis Registry; has been an investigator for AbbVie, Cara, Corrona Psoriasis Registry, Dermavant, Dermira, and Novartis; and is Editor-in-Chief (honorarium) for the Journal of Psoriasis and Psoriatic Arthritis. Prof Warren has received research grants and/or consulting fees from AbbVie, Almirall, Amgen, Arena, Avillion, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB Pharma. Prof Reich has served as advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Covagen, Dermira, Eli Lilly, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Medac, MSD, Miltenyi Biotec, Novartis, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB Pharma, Valeant/Bausch Health, and Xenoport.
Acknowledgements: Medical writing assistance was provided by Dr Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Support: This article was funded by an educational grant from UCB Pharma, who had no input into its content. The views and opinions expressed are those of the speakers.
Citation: EMJ Dermatol. 2020;8[1]:36-43.
Meeting Summary
Prof Strober introduced the Phase III BE VIVID trial in which the efficacy of bimekizumab (BKZ) was compared with that of ustekinumab (UST) and placebo (PBO) in patients with moderate-to-severe plaque psoriasis. He described how BKZ provided robust and durable complete skin clearance through 52 weeks of treatment, as shown by higher Psoriasis Area and Severity Index (PASI) 100 responses compared with UST, regardless of baseline demographics, disease characteristics, or prior treatment exposure. Prof Warren discussed the results of the Phase III BE SURE trial that evaluated efficacy and safety of BKZ versus adalimumab (ADA) in patients with moderate-to-severe plaque psoriasis. He highlighted how BKZ was associated with superior levels of skin clearance compared with ADA, with durable clinical responses through Week 56, regardless of the BKZ maintenance dose. Switching from ADA to BKZ resulted in rapid increases in PASI 90, PASI 100, and Investigator’s Global Assessment (IGA) 0/1 responder rates, with results comparable to BKZ-randomised patients at Week 56. BKZ was generally well tolerated, with treatment-emergent adverse events (TEAE) as expected for the mode of action and comparable with previous studies. Prof Reich summarised the pooled safety data from eight Phase II and III clinical trials in patients with moderate-to-severe plaque psoriasis and explained that BKZ was well tolerated across the psoriasis clinical programme. The majority of TEAE were mild-to-moderate and discontinuation rates were low. Candida infections with BKZ were expected considering the mode of action of this drug (IL 17 inhibition). All candida infections were mucocutaneous in origin; oral candidiasis was the most common. Oral candidiasis TEAE were predominantly mild-to-moderate, easily treated, and did not lead to discontinuation.
Bimekizumab Versus Ustekinumab Efficacy Across Subgroups of Patients with Moderate-to-Severe Plaque Psoriasis: Results from the Multicentre, Randomised, Double-Blinded Phase III BE VIVID Trial1
Professor Bruce Strober
Psoriasis is a Th17-driven disease, with both IL-17A and IL-17F playing a pivotal role in its pathogenesis.2,3 BKZ is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F by binding with high affinity to a similar site on both isoforms.4,5
Severity of psoriasis and response to psoriasis therapies can vary with patient age, weight, prior treatment exposure, and other factors.6 Therapies that provide a consistent and durable response regardless of these variables are needed, and it is important to understand how the efficacy of psoriasis therapies on skin clearance may differ between patients.
BE VIVID7 was a randomised, double-blinded, PBO- and active comparator-controlled, Phase III trial to compare the efficacy of BKZ with that of UST over 52 weeks of treatment in adult patients with moderate-to-severe plaque psoriasis. Patients were randomised 4:2:1 to BKZ 320 mg every 4 weeks (q4w) through Week 52, UST 45 mg/90 mg (by weight) every 12 weeks through Week 52, or PBO. Patients randomised to PBO switched treatment at Week 16 to receive BKZ 320 mg q4w through Week 52. Randomisation was stratified by prior biologic exposure and region.
The authors conducted post hoc analyses of the following subgroups including only those patients who were randomised to BKZ or UST: baseline weight (≤100, >100 kg), prior biologic exposure, prior anti-TNF exposure, prior anti-IL-17 exposure, prior anti-IL-23 exposure, age (<40, 40–<65, ≥65 years), psoriasis disease duration (<median [14.54 years] or ≥median), baseline disease severity (absolute PASI: <20 or ≥20), and baseline IGA 3 or 4. Proportions of BKZ- versus UST-treated patients who achieved PASI 90 and PASI 100 were calculated at Weeks 16 and 52.
The authors reported that baseline characteristics were well balanced in the BKZ (n=321) and UST (n=163) treatment groups: baseline mean ± standard deviation PASI was 22.0±8.6 and 21.3±8.3, and body surface area affected was 29.0±17.1% and 27.3±16.7%, respectively. The duration of psoriasis was 16.0±11.6 years and 17.8±11.6 years in the BKZ and UST groups, respectively, and just over one-third of patients had previously been exposed to biologic therapy (125/321 [38.9%] in the BKZ group and 63/163 [38.7%] in the UST group).
Overall, at Week 16, PASI 90 was achieved by 85.0% and 49.7% of patients randomised to BKZ and UST, respectively, with results at Week 52 of 81.9% and 55.8%, respectively. Results were consistent across the patient subgroups at both time points.
At Weeks 16 and 52, respectively, 58.6% and 64.5% of patients who received BKZ and 20.9% and 38.0% who received UST achieved PASI 100. A high level of PASI 100 response to BKZ was seen across all subgroups at both Week 16 and Week 52 (Table 1). The proportion of patients who achieved PASI 100 at Week 16 was greater for patients randomised to BKZ compared with UST across all subgroups (44.1–63.6% for BKZ and 0.0–29.2% for UST). Among BKZ-treated patients at Week 16, 60.2% (136/226) weighing ≤100 kg and 54.7% (52/95) weighing >100 kg achieved PASI 100 (compared with 23.0% and 14.6%, respectively, with UST), as did 60.8% (76/125) with and 57.1% (112/196) without prior biologic exposure (compared with 22.2% and 20.0%, respectively, with UST). PASI 100 responses at Week 16 were further improved at Week 52, with 50.0–69.1% of patients across subgroups who received BKZ and 16.7–50.9% of patients across subgroups who received UST achieving PASI 100.
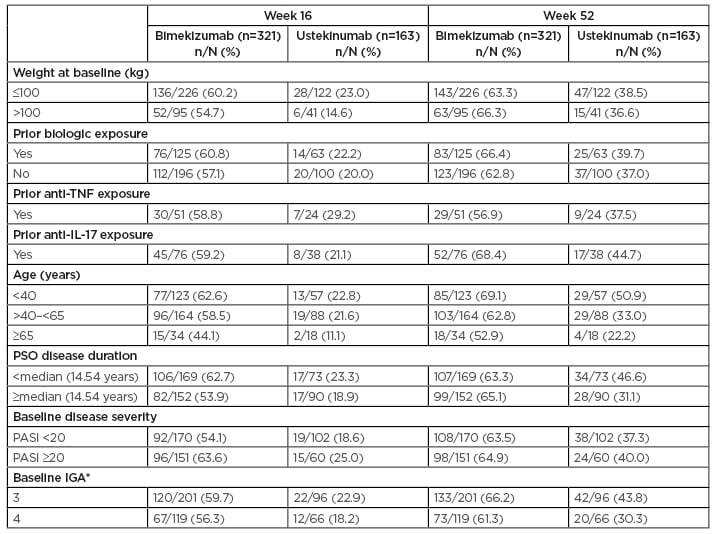
Table 1: Patients achieving PASI 100 among subgroups (nonresponder imputation).
Nonresponder imputation was used for all missing data.
* One patient in the bimekizumab group and one patient in the ustekinumab group had a baseline IGA=2 and are not included here.
IGA: Investigator’s Global Assessment; PASI: absolute Psoriasis Area and Severity Index; PSO: moderate-to-severe psoriasis.
Reproduced with permission from UCB within the context of the independent grant request, and with permission from Warren et al.8
Among BKZ-treated patients at Week 52, 63.3% (143/226) weighing ≤100 kg and 66.3% (63/95) weighing >100 kg achieved PASI 100 (compared with 38.5% and 36.6%, respectively, with UST), as did 66.4% (83/125) with and 62.8% (123/196) without prior biologic exposure (compared with 39.7% and 37.0%, respectively, with UST).
The authors concluded that BKZ provided robust and durable complete skin clearance in patients with moderate-to-severe plaque psoriasis through 52 weeks, regardless of baseline demographics, disease characteristics, or prior treatment exposure. At Week 16, a greater proportion of BKZ-treated patients achieved PASI 90 and PASI 100 compared with UST-treated patients in all subgroups. Responses were further improved or maintained for BKZ through Week 52 and remained higher than responses with UST. These results were considered by the authors to support BKZ as a psoriasis treatment suitable for a wide variety of patients, given its consistent efficacy across all subgroups analysed.
Bimekizumab Efficacy and Safety Versus Adalimumab in Patients with Moderate-to- Severe Plaque Psoriasis: Results from a Multicentre, Randomised, Double-Blinded Active Comparator-Controlled Phase III Trial (BE SURE)8
Professor Richard Warren
BE SURE9 was a randomised, double-blinded, active comparator-controlled Phase III trial to evaluate the efficacy and safety of BKZ versus ADA in adult patients with moderate-to-severe plaque psoriasis, and to assess the maintenance of efficacy of BKZ dosed in two different regimens. Patients were randomised 1:1:1 to BKZ 320 mg q4w for 56 weeks, BKZ 320 mg q4w for 16 weeks followed by BKZ 320 mg every 8 weeks (q8w) to Week 56, or ADA 40 mg every 2 weeks (q2w) for 24 weeks followed by BKZ 320 mg q4w to Week 56. Co-primary endpoints were PASI 90 and IGA 0/1 versus ADA at Week 16. Secondary endpoints included PASI 90 and IGA 0/1 at Weeks 24 and 56, and PASI 100 at Weeks 16 and 24.
A total of 158, 161, and 159 patients were randomised to BKZ 320 mg q4w, BKZ 320 mg q4w/q8w, or ADA 40 mg q2w/BKZ 320 mg q4w, respectively. Baseline demographics and characteristics were comparable in the three groups. Disease duration was long, with mean ± standard deviation of 20.4±13.2, 17.3±10.9, and 16.2±11.9 years with BKZ 320 mg q4w, BKZ 320 mg q4w/q8w, and ADA 40 mg q2w/BKZ 320 mg q4w, respectively. Approximately one-third of patients in each group had prior biologic therapy (50/158 [31.6%], 50/161 [31.1%], and 53/159 [33.3%] in the BKZ 320 mg q4w, BKZ 320 mg q4w/q8w, and ADA 40 mg q2w/BKZ 320 mg q4w groups, respectively).
The authors reported that all primary and ranked secondary endpoints were achieved. At Week 16, significantly more patients achieved PASI 90 and IGA 0/1 with BKZ (86.2% and 85.3%, respectively) than with ADA (47.2% and 57.2%, respectively), and PASI 100 was achieved by 60.8% of patients who received BKZ versus 23.9% who received ADA (all comparisons: p<0.001) (Table 2).8 PASI 90 and PASI 100 response rates within BKZ treatment arms were durable through Week 56, irrespective of maintenance dose. Notably, using a nonresponder imputation analysis, 71.2% of patients who received BKZ achieved the PASI 100 threshold at Week 56 (Table 2).
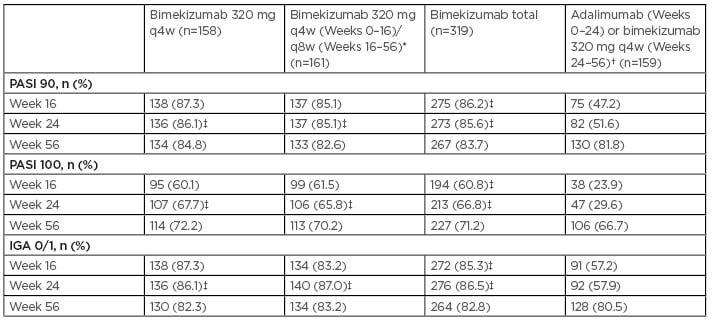
Table 2: PASI 90, PASI 100, and IGA 0/1 responses in patients randomised to receive bimekizumab and adalimumab (switching to bimekizumab at Week 24) though Week 56 (nonresponder imputation).
* Patients received bimekizumab 320 mg q4w for 16 weeks followed by q8w through Week 16–56.
† Patients randomised to adalimumab switched to bimekizumab 320 mg q4w at Week 24 (10 patients randomised to adalimumab at Week 0 did not continue in the trial past Week 24 and never received bimekizumab).
‡ p value versus adalimumab p<0.001.
PASI 90/100: ≥90/100% improvement from baseline in PASI.
IGA assessed on a five-point scale.
Data shown include all randomised patients. Missing data were imputed as nonresponder imputation. p values for the comparison of treatment groups are based on the Cochran–Mantel–Haenszel test from the general association.
IGA: Investigator’s Global Assessment; PASI: Psoriasis Area and Severity Index; q4w: every 4 weeks; q8w: every 8 weeks.
Reproduced with permission from UCB within the context of the independent grant request, and with permission from Warren et al.8
In patients randomised to ADA, PASI 90, PASI 100, and IGA 0/1 responder rates rapidly increased following the switch to BKZ 320 mg q4w at Week 24. At Week 56, responder rates were comparable with those in patients who received BKZ continuously from Week 0.
The authors explained that TEAE and serious TEAE were comparable for patients who received BKZ (71.5% and 1.6%, respectively) and those who received ADA (69.8% and 3.1%, respectively) during Weeks 0 to 24. A total of 81.4% and 5.1% of patients who received BKZ (including those who switched from ADA) experienced TEAE and serious TEAE, respectively, during Weeks 0 to 56.
There were no unexpected safety findings in patients who switched from ADA to BKZ compared with patients who received continuous BKZ treatment.
There was one death in the study in a patient who received ADA; this was unrelated to treatment. The most common TEAE in the study was nasopharyngitis (which is a common side effect in biologic studies), with generally comparable incidence in the different treatment groups (20.3%, 16.8%, and 23.9% in the BKZ 320 mg q4w, BKZ 320 mg q4w/q8w, and ADA 40 mg q2w/BKZ 320 mg q4w groups, respectively, at Weeks 0–24, and 11.8%, 10.1%, and 13.4%, respectively, at Weeks 24–56). Oral candidiasis occurred in approximately 10% of patients in the BKZ groups compared with 0% in the ADA group at Weeks 0–24; however, cases of oral candidiasis were mostly mild or moderate, localised, and easily treatable and did not lead to discontinuation. Over 56 weeks, there were no cases of suicidal ideation or behaviour, inflammatory bowel disease, serious hypersensitivity reactions, or major adverse cardiac events in BKZ-treated patients.
The authors concluded that BKZ q4w was associated with superior levels of skin clearance compared with ADA, with complete skin clearance (PASI 100) at Week 16 achieved by 61% of patients who received BKZ q4w versus 24% who received ADA. Clinical responses were durable through Week 56, regardless of the BKZ q4w or q8w maintenance dose. Switching from ADA to BKZ resulted in rapid increases in PASI 90, PASI 100, and IGA 0/1 responder rates, with results comparable to BKZ-randomised patients at Week 56. There were no new safety signals with BKZ, which was generally well tolerated, and results were comparable with previous studies.
Bimekizumab Safety in Patients with Moderate-to- Severe Psoriasis: Analysis of Pooled Data from Phase II and III Clinical Trials10
Professor Kristian Reich
BKZ has a new mode of action: in addition to blocking IL-17A (like secukinumab and ixekizumab) it blocks IL-17F; therefore, it is important to assess the safety data associated with this new mode of action as early as possible. Furthermore, psoriasis is a chronic disease requiring long-term management, so it is of interest to ascertain the safety profile of therapies such as BKZ. Pooled data from eight Phase II and III clinical trials were analysed to derive short and longer-term safety data in BKZ-treated adult patients with moderate-to-severe plaque psoriasis.
Safety from the initial treatment periods (Weeks 0–16) of three Phase III trials (BE SURE, BE VIVID, and BE READY11) was evaluated for patients who received ≥1 dose of BKZ, UST, ADA, or PBO. Longer-term safety was also evaluated for patients who received ≥1 dose of UST through 52 weeks in BE VIVID, and for patients who received ≥1 dose of BKZ in BE SURE, BE VIVID, BE READY, the BE BRIGHT12 open-label extension Phase III trial (interim cut-off: 1st Nov 2019), and four Phase II trials (BE ABLE 1,13 BE ABLE 2,14 PS0016,15 and PS001816).
A total of 989 patients received ≥1 BKZ dose for 16 weeks, representing 306.0 patient-years (PY) of exposure, 163 patients received UST (50.1 PY), 159 received ADA (48.8 PY), and 169 received PBO (51.6 PY). Mean psoriasis duration in the study patients was >16 years. Around one-third of the patients had received prior biologic therapy (38.4% on BKZ 320 mg q4w, 33.3% on ADA, 38.7% on UST, and 41.4% on PBO).
During the initial 16 weeks of treatment, the authors reported that at least one TEAE was experienced by 593 (60.0%) patients on BKZ, 83 (50.9%) on UST, 96 (60.4%) on ADA, and 74 (43.8%) on PBO, with no significant trend noted. Serious TEAE were reported in 15 (1.5%) patients on BKZ, five (3.1%) on UST, three (1.9%) on ADA, and four (2.4%) on PBO. Treatment discontinuation as a result of TEAE occurred in 17 (1.7%) patients on BKZ, three (1.8%) on UST, four (2.5%) on ADA, and seven (4.1%) on PBO. One death occurred in each treatment group.
Focussing on TEAE of special interest during the initial treatment period (Weeks 0–16), serious infections occurred in three (0.3%) BKZ-treated patients, oral candidiasis in 75 (7.6%), and de novo ulcerative colitis in one (0.1%). The candida infections with BKZ were expected considering the mode of action of this drug (IL-17 inhibition).
The authors highlighted that exposure-adjusted incidence rate per 100 PY of selected TEAE and TEAE of special interest generally did not increase with BKZ exposure duration (Table 3).
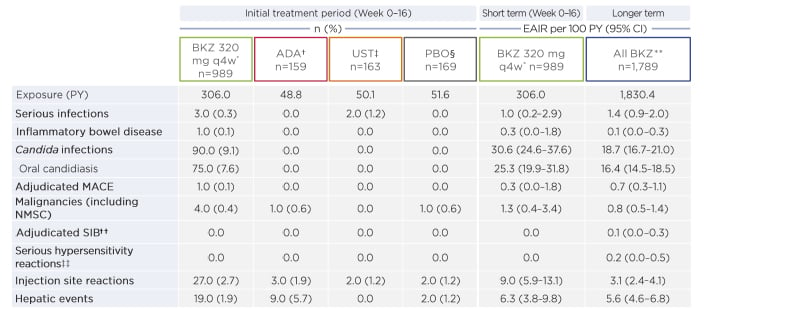
Table 3: Treatment-emergent adverse events of interest (short and longer term).
EAIR are patient incidence of new cases per 100 PY.
* BKZ initial treatment period data are included from three pivotal Phase III studies.
† ADA initial treatment period data are from BE SURE.
‡ UST initial treatment period data are from BE VIVID.
§ PBO initial treatment period data are from BE VIVID and BE READY.
** BKZ longer-term data are pooled from four Phase III trials and four Phase II trials.
†† Includes one event adjudicated by the external Neuropsychiatric Committee (active suicidal ideation with some intent to act) in a patient with pre-existing psychiatric conditions.
‡‡ Includes one fatal event of circulatory failure (adjudicated MACE), one event of atopic dermatitis-like disseminated eczema, and one case of anaphylactic shock due to insect sting, all considered unrelated to study treatment.
ADA: adalimumab; BKZ: bimekizumab: CI: confidence interval; EAIR: exposure-adjusted incidence rate; MACE: major adverse cardiovascular event; NMSC: nonmelanoma skin cancers; PBO: placebo; PY: patient-years; q4w: every 4 weeks; SIB: suicidal ideation and behaviour; UST: ustekinumab.
Reproduced with permission from UCB within the context of the independent grant request, and with permission from Warren et al.8
Over the long term, TEAE in BKZ-treated patients occurred at a rate (95% confidence interval [CI]) of 238.0 (226.0–250.5)/100 PY, serious TEAE at 6.6 (5.5–7.9)/100 PY, and discontinuations because of TEAE at 4.9 (4.0-6.1)/100 PY. Five deaths occurred (0.3 [95% CI: 0.1–0.6]/100 PY), all of which were unrelated to treatment.
A total of 304 (17.0%) BKZ-treated patients had mucocutaneous candida infection TEAE (18.7 [95% CI: 16.7–21.0]/100 PY). Of the 304, 271 (15.1%) had oral candidiasis (16.4 [95% CI: 14.5–18.5]/100 PY). Most candidiasis cases (>99%) were mild-to-moderate and easily treated and did not lead to discontinuation. One (<0.1%) serious case (oesophageal candidiasis) and 6 (0.3%) discontinuations as a result of candidiasis were reported.
There were low rates of malignancy (0.8 [95% CI: 0.5–1.4]/100 PY) and adjudicated major adverse cardiac events (0.7 [95% CI: 0.3–1.1]/100 PY) in patients who received BKZ. There was one case of active suicidal ideation (0.1/100 PY) in a BKZ-treated patient with a prior history of suicide attempt. There were no cases of anaphylaxis and no additional cases of inflammatory bowel disease with increased exposure to BKZ.
The majority of TEAE were mild-to-moderate and discontinuation rates were low. There were candida infections, as expected for this class (IL-17 inhibitors). Overall, the exposure-adjusted incidence rate of TEAE and TEAE of interest did not increase with BKZ exposure. The authors concluded that BKZ was well tolerated across the psoriasis clinical programme, with no new safety signals compared to other targeted therapies.







