Disclosure: The European Medical Group has engaged in business transactions with Janssen.
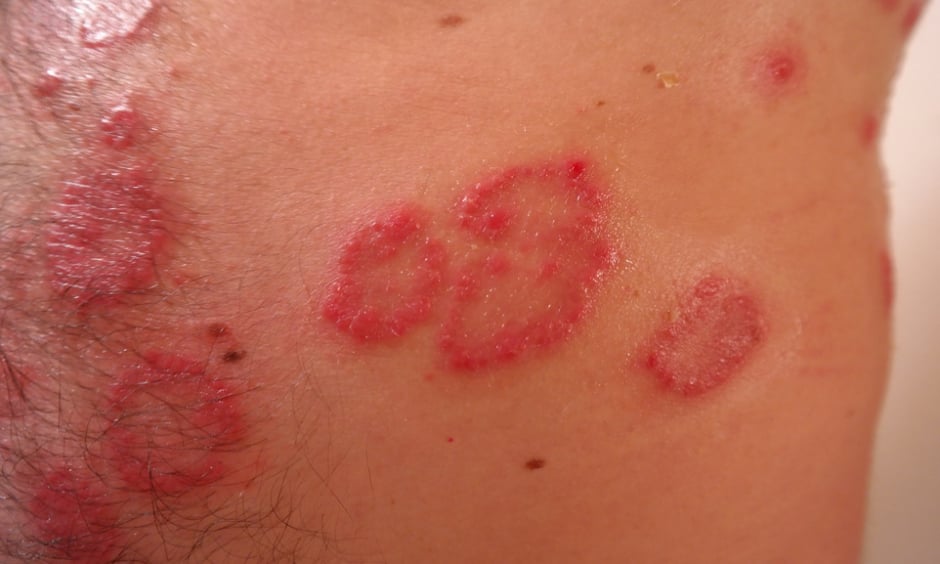
GUSELKUMAB (Tremfya®, Janssen, Beerse, Belgium) has been recommended for the treatment of adults with moderate-to-severe plaque psoriasis by the UK’s National Institute for Health and Care Excellence (NICE). The Final Appraisal Determination (FAD) was fast-tracked following approval given by the European Commission last November for guselkumab to treat this condition.
Positive Trial Results
The decision by the European Commission was based on the results of several Phase 3 studies, including the VOYAGE 1 and 2 clinical trials, in which guselkumab was compared to placebo and adalimumab. In these studies, a Psoriasis Area and Severity Index (PASI) score of 90 was achieved in 73.3% and 70.0% of guselkumab-treated patients compared with 49.7% and 46.8% of those receiving adalimumab, respectively, after 16 weeks. Additionally, consistent skin clearance rates were observed in patients treated with guselkumab through Week 100.
NICE Recommendation
The recommendation from NICE qualifies the use of guselkumab for plaque psoriasis in adults when the severity of the disease is 10 or above on PASI and over 10 on the Dermatology Life Quality Index (DLQI); other systemic therapies, such as ciclosporin and methotrexate, have been unable to produce a response, are not tolerated, or contraindicated; and the drug is provided by the company with the discount agreed in the patient access scheme.
Important Advance
With psoriasis affecting around 14 million people across Europe, it is vital that new treatments are developed that improve long-term outcomes for patients. “Psoriasis is a serious long-term condition with important comorbidities that can impact patients’ daily lives,” commented Prof Chris Griffiths, University of Manchester, Manchester, UK, who was a Steering Committee member of the VOYAGE 1 study. “Guselkumab provides a significant and welcome advance in our management of psoriasis with a high percentage of patients achieving clear or nearly clear skin over the long-term.”
Adverse Events
During the first 16 weeks of VOYAGE 1 and 2, adverse events were reported in at least 5% of patients treated with guselkumab. These included nasopharyngitis, upper respiratory tract infection, and back pain, and the types reported were generally consistent though 48 weeks of treatment.
Self-Administration
An injectable form for psoriasis, guselkumab can be self-administered by patients following training.
James Coker, Reporter