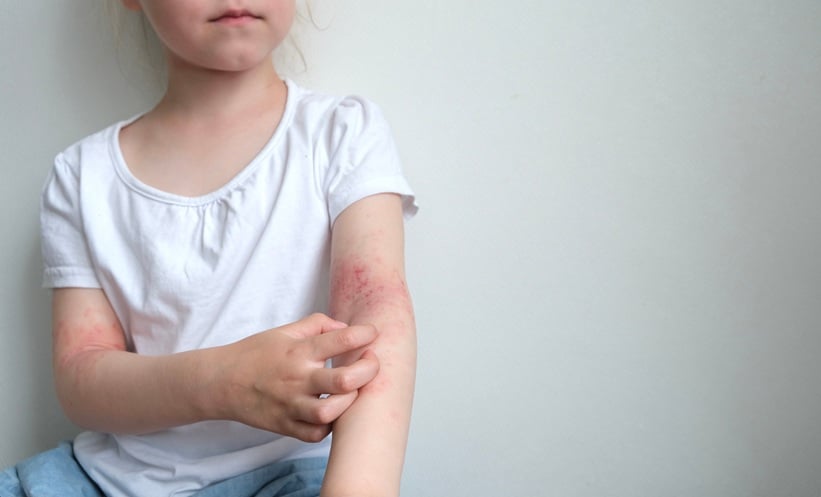
A RECENT Japanese study has shown that a newly developed skincare treatment, partially myristoylated carboxymethyl chitosan (PMCMC) emulsion, may offer a more effective alternative to traditional petrolatum in managing mild atopic dermatitis in young children. In a randomised controlled trial involving 80 children aged between one and six years, researchers compared the efficacy and safety of PMCMC-containing emulsion with petrolatum over an eight-week treatment period.
Participants, all with mild atopic dermatitis, were randomly assigned to apply either PMCMC emulsion or petrolatum to their bodies twice daily. The study focused on two main outcomes: changes in disease severity, measured by the Eczema Area and Severity Index (EASI), and the quality of life of caregivers, assessed using the Quality of life in Primary Caregivers of children with Atopic Dermatitis (QP-CAD) score.
Results revealed that the group using PMCMC emulsion experienced a significantly greater reduction in EASI scores compared to the petrolatum group. The average decrease in disease severity was −0.74 for the emulsion group versus an increase of 0.29 in the petrolatum group, with a statistically significant difference. In terms of caregiver quality of life, the emulsion group also saw greater improvements, with a reduction of −1.63 in QP-CAD scores, compared to a rise of 1.55 in the petrolatum group. Again, this difference was statistically significant.
Importantly, all adverse events reported in the PMCMC group were mild, suggesting that the treatment is not only effective but also safe for young children. This trial highlights PMCMC-containing emulsion as a promising new option for managing mild atopic dermatitis, offering benefits not only for affected children but also for their caregivers. Further research may help to confirm these findings and support broader use of this innovative formulation.
Reference
Sagara N et al. Emulsion containing a chitosan derivative in children with mild atopic dermatitis: a randomized controlled trial. Pediatr Allergy Immunol. 2025;36(4):e70076.