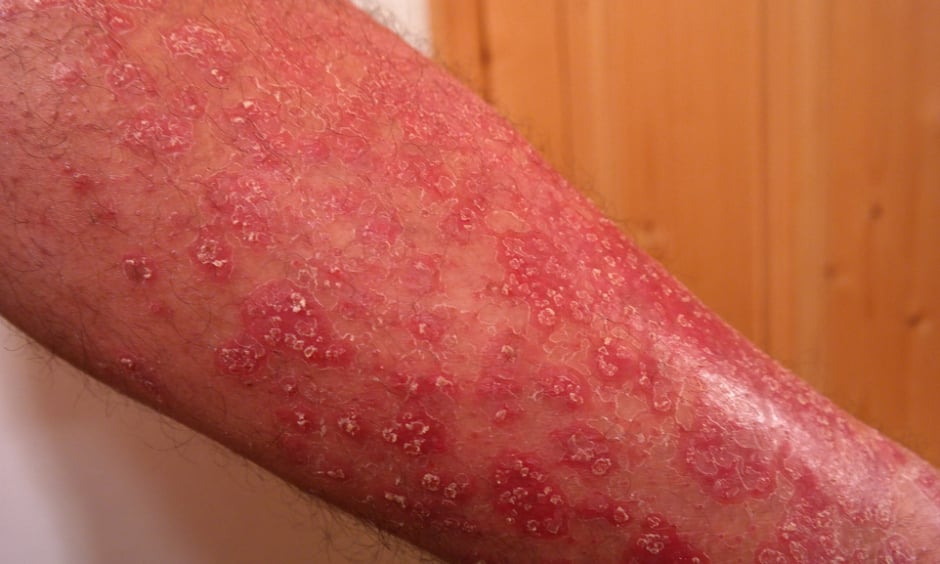
PSORIASIS treatment could be revolutionised following the recent approval of an interleukin-23 antagonist, known as tildrakizumab, by the U.S. Food and Drug Administration (FDA). The drug, which will be marketed as Ilumya, has been approved to treat patients with moderate-to-severe plaque psoriasis who are eligible for systemic therapy or phototherapy.
FDA approval of tildrakizumab was achieved following the outcome of two identically designed multicentre, randomised, double-blind, placebo-controlled Phase III clinical trials, reSURFACE1 and reSURFACE2. During the studies, 926 patients were enrolled: 616 patients received a 100 mg dose of tildrakizumab subcutaneously at Week 0, Week 4, and then every 12 weeks, and 310 patients were given a placebo.
At Week 12 of the study, after receiving two doses, both patient groups were assessed using the Psoriasis Area and Sensitivity Index (PASI) and a Physician’s Global Assessment (PGA) score. The two clinical trials showed the beneficial effect of tildrakizumab administration on achieving PASI 75 and improving PGA score compared to placebo: a PASI 75 score was achieved by 64% and 61% of tildrakizumab patients in the two trials, compared to 6% observed in both control groups. Furthermore, a PGA score of 0 or 1 was recorded in 58% and 55% of tildrakizumab patients, whereas 7% and 4% of the control groups had a PGA score of 0 or 1 in each trial. Tildrakizumab also proved to be effective beyond the initial 12-week time period; by Week 64, 84% of patients that were receiving tildrakizumab had achieved a PASI 75 score.
Since it has been noted that tildrakizumab can induce serious side effects in some patients, including serious allergic reactions, approval of the drug was given alongside a medication guide to inform patients of the potential risks. Tildrakizumab could yet provide an effect therapeutic option for psoriasis, and its availability will be determined once postapproval commitments by the FDA are complete.