Abstract
Cutaneous reactions following the COVID-19 vaccination, in particular mRNA vaccines, have been increasingly reported in literature. The most common morphologies were delayed large local reactions, local injection site reactions, urticaria, and morbilliform reaction. The purpose of this report is to review the cutaneous manifestations of COVID-19 vaccines and to report two cases of COVID-19 mRNA vaccine-induced varicella zoster reactivation along with the possible pathogenesis.
These two cases are of an 80-year-old female patient with multiple comorbidities and a previously healthy 57-year-old male patient who experienced varicella zoster reactivation post-COVID-19 mRNA vaccine (Pfizer, New York City, New York, USA) following the first and second dose.
INTRODUCTION
Cutaneous reactions following COVID-19 vaccination, in particular mRNA vaccines, have been increasingly reported in literature. The most common side effects encountered during the worldwide administration of the various type of COVID-19 vaccinations ranged from mild local injection site reactions to more severe urticaria and morbilliform reactions. The less common side effects included varicella zoster reactivation post-vaccination.1
Here, the authors report two cases of varicella zoster reactivation after a COVID-19 mRNA vaccine.
CASE 1
An 80-year-old female patient with hypertension, Type 2 diabetes, hypothyroidism, dyslipidaemia, history of a gastrointestinal stromal tumour, and overactive bladder presented with 3 days history of a painful erythematous vesicular eruption on the left thigh. The patient reported that they began to experience generalised myalgias and fatigue 4 days after the first dose of their COVID-19 vaccine (Pfizer [New York City, New York, USA] and BioNTech [Mainz, Germany] vaccine), and low-grade fever followed 1 day later by the eruption of multiple fluid-filled vesicles on their left thigh, which were painful and itchy. A review of system was negative. The patient was not on any immunosuppressive drugs, had no recent trauma or surgery, had no previous history of varicella or shingles, and was not exposed to anyone with varicella or zoster infection.
A physical exam revealed the presence of a cluster of clear, pus-filled vesicles that were surrounded by erythema and covered an area on the left thigh corresponding to the L2 and L3 dermatomes in a linear distribution (Figure 1A).
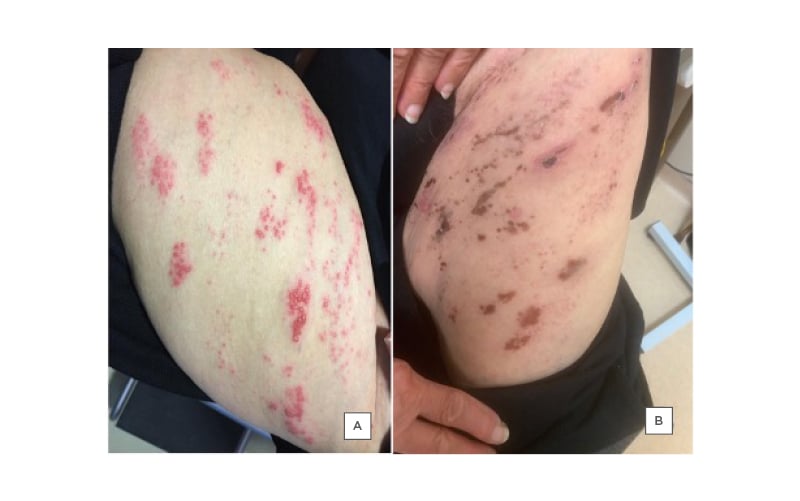
Figure 1: Fluid-filled vesicles on the left thigh (L2–L3 dermatomes) of an 80-year-old female as a result of varicella zoster.
A) Shows the patient before and B) shows the patient after their treatment.
A clinical diagnosis of herpes zoster (HZ) was made, and the patient was started on valacyclovir 500 mg, two tablets thrice a day for 7 days, with daily antiseptic wash and painkillers.
The patient presented for follow-up after 1 week with slight improvement; some of the vesicles were dried out while others were still filled with pus (Figure 1B). The patient was started empirically on cefadroxil 500 mg, two tablets twice daily for 3 days, and then one tablet twice daily for 6 days. Tzanck smear (Figure 2), PCR, and bacterial cultures were completed from the fluid- and pus-filled vesicles. The smear results were positive for Tzanck cells and varicella zoster virus (VZV) was detected on PCR, while the bacteriological cultures were negative.
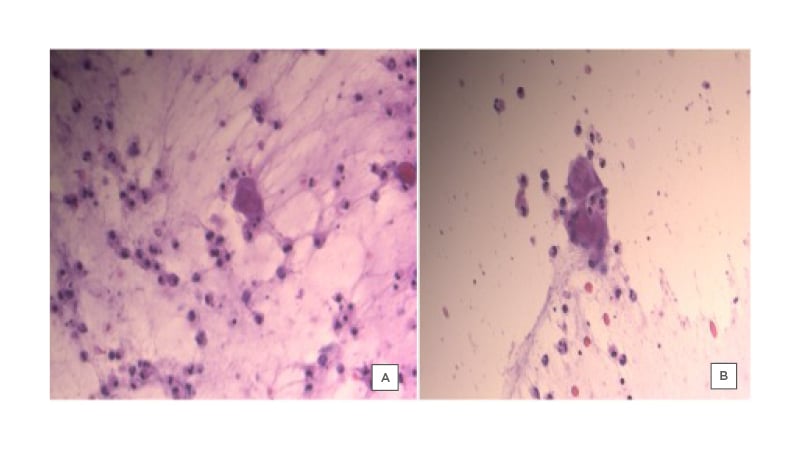
Figure 2: Tzanck smear from vesicles of a female patient, showing the presence of multinucleated giant cells.
Follow-up after 6 days showed an improvement of the symptoms, with the vesicles crusting.
The patient was advised to delay the second dose of the vaccine until full resolution of the rash.
CASE 2
A 57-year-old male patient, with no known medical conditions, presented with a 5-day history of a vesicular eruption on the left thoracic area along the T3–T4 dermatomes.
The patient reported that 2 days after receiving their second dose of the COVID-19 vaccine (Pfizer Inc. and BioNTech vaccine), they noticed the appearance of multiple clusters of vesicular lesions on their chest in a linear distribution over T2–T4 dermatomes (Figure 3), which was associated with a burning pain and stinging sensation. The patient denied the presence of any associated symptoms. They were not on any immunosuppressive drugs, had no recent trauma or surgery, had no previous history of varicella or shingles infection, and were not exposed to anyone with varicella or zoster infection. After the appearance of the vesicular eruption on the chest area, the patient followed up with their physician and a clinical diagnosis of HZ was made. The patient was started on valacyclovir 500 mg, two tablets thrice a day for 7 days, with a daily antiseptic wash.
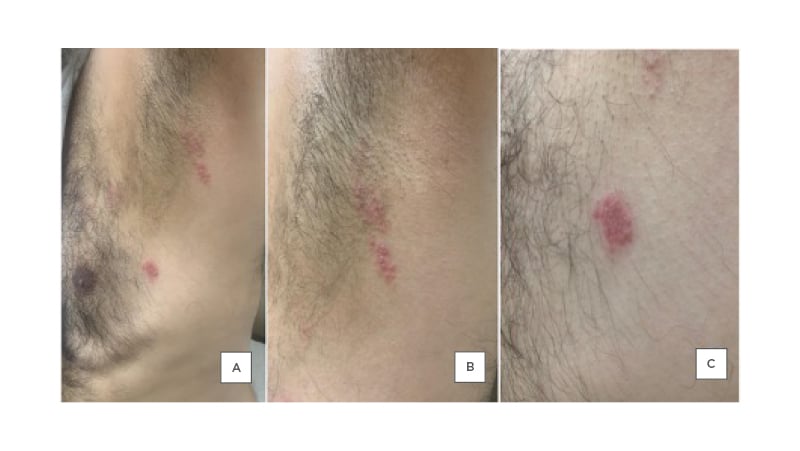
Figure 3: Multiple vesicular lesions on the chest of 57-year-old male.
On follow-up 7 days later, the patient reported that the lesions had crusted over, and the pain associated with the rash was substantially improved.
DISCUSSION
The cutaneous manifestations of COVID-19 are increasingly being reported in the literature.2
The VZV is responsible for the development of varicella and shingles. It is transmitted either by the airborne route or through direct contact with the vesicles.3
Following primary infection, the virus remains latent in the sensory dorsal root ganglion. Reactivation of the virus can be triggered by various factors, including stress, old age, immunocompromised status, as well as various immunosuppressive drugs.3
After the introduction of COVID-19 vaccines and the rapid spread of vaccination campaigns, light is shed on the cutaneous manifestations associated with the COVID-19 vaccines. To date, three main types of vaccines are being utilised in the world for COVID-19 and these include mRNA-based vaccines: mRNA-1273 (Moderna, Cambridge, Massachusetts, USA) and BNT162b2 (Pfizer and BioNTech); adenoviral vector vaccines: ChAdOx1 nCOV-19 (Oxford–AstraZeneca, Cambridge, UK), Sputnik V or Gam-COVID-Vac (Gamaleya Research Institute of Epidemiology and Microbiology, Moscow, Russia), and Ad26.COV2.S (Janssen, Beerse, Belgium); and inactivated whole virus vaccines: BBIBP-CorV (Sinopharm, Beijing, China) and CoronaVac (Sinovac, Beijing, China).4
Injection site reactions were the most frequent side effects described in all three types of vaccines. They were due to non-specific stimulation of inflammation. These reactions ranged from mild injection site reactions, redness, and swelling, to injection site urticaria and maculo-papular dermatitis.4 The underlying mechanism causing vaccine-related reactions are thought to be a Type 1 IgE-mediated hypersensitivity reaction to various excipients of the vaccines.4 Other than the acute injection site reaction, a delayed Type 4 hypersensitivity reaction can also occur with the vaccines. This is seen with the mRNA vaccines, where erythema, tenderness, and induration appear after a week on average post-vaccine. Both the acute and delayed hypersensitivity reactions are non-severe and can be managed symptomatically.4 Anaphylactic reactions are the more serious adverse events that can be associated with the vaccines. In the case of the mRNA vaccines, such reactions are induced by polyethylene glycol and macrogol.4 For the other types of vaccines, including the AstraZeneca and Janssen ones, cutaneous reactions have linked them with the possible vaccine-induced prothrombotic immune thrombocytopenia. Vaccine-induced prothrombotic immune thrombocytopenia can manifest with petechial eruption or erythema along with the associated systemic symptoms involving the nervous, cardiovascular, and the gastrointestinal systems.4 Other reported skin findings associated with the AstraZeneca vaccine include injection site reaction, pruritic erythematous maculopapular eruption, and one case of cellulitis.4
As for the Russian vaccine, Sputnik V, an adenoviral-based two-part vaccine, reactions ranged from mild injection site reactions to hives in one patient. Other reactions included petechial rash, extremity abscess, acneiform dermatitis, eczema, and alopecia.4
The Chinese vaccine, CoronaVac, an inactivated vaccine, mostly showed injection site reactions. Other rare events include non-infectious gingivitis, buccal ulcerations, oral herpes simplex virus, and lymphadenopathy.4
A few reports emerged linking the COVID-19 infection to HZ reactivation in adults, some of whom presented with severe necrotic zoster.5-8 On the other hand, there are very scarce reports of HZ reactivation post-vaccine, despite the high rate of vaccination worldwide. Seven cases were reported after an mRNA vaccine (one case report and six patients in an observational study), temporally linking the COVID-19 vaccine with the development of HZ.9,10
To date, no clear mechanism has been identified for the development of HZ post-COVID-19 infection or vaccination. The proposed mechanism underlying reactivation of HZ in patients infected with COVID-19 is secondary to a drastic decrease in the number of lymphocytes, mainly with reduced numbers of cluster of differentiation 4 positive T cells, cluster of differentiation 9 positive T cells, B cells, and natural killer cells, as well as eosinophils and monocytes.11
Concerning the link between HZ reactivation and COVID vaccine, van Dam et al.12 also reported lymphopenia to occur in a dose-dependent manner in vaccinated patients, reaching its peak a few days following vaccination, then progressively returning to normal 6–8 days post-vaccination; it has therefore been proposed that, during this window, a VZV reactivation could take place. Other factors that might be implicated include both physical and psychological stress.12 Other plausible mechanisms that have been suggested are attributed to immune dysregulation.10 According to Furer et al.,9 mRNA COVID-19 vaccines stimulate the innate immunity through toll-like receptors, which are essential for reactivation of herpes viruses. Several studies have reported that toll-like receptor reactivation can function as a signal that triggers lytic replication of viral particles within the host cells and, thus, cause the reactivation of the virus.13 This theory of immune dysregulation has been observed and speculated with VZV reactivation after vaccines such as hepatitis A, hepatitis B, and influenza, with the exact mechanism not clearly elucidated.14
Multiple risk factors have been implicated in the reactivation of HZ infection, including older age, immune compromise, and stress.3 In the authors’ cases, the only risk factor was older age in the first case. The absence of immunosuppression or a clear trigger factor, as well as the temporal association of the symptoms and vaccine receipt, allowed the authors to presume the presence of a connection between virus reactivation and the vaccine in both cases.
As for whether the authors’ patients should receive the second dose of the vaccine or not, this remains a subject of debate. In the literature, 43% of patients who received the mRNA vaccine experienced cutaneous adverse effects after the second dose, such as delayed large local reactions, local injection site reactions, morbilliform rash, and urticaria. Those cutaneous reactions were reported to be similar to the initial reaction after the first dose of vaccine in 28%, milder in 28%, and more robust in 45% of these patients.1 As HZ reactivation was not the consequence of a direct immune-mediated reaction to the vaccine in these cases, the authors encouraged the patients to take the second dose; however, they refused to do so.
CONCLUSION
In conclusion, with the rapid administration of COVID-19 vaccines worldwide, recognition of its possible dermatological side effects should be recognised. The authors’ cases represent one of the possible cutaneous manifestations associated with the COVID-19 vaccine, in particular the mRNA vaccine.






