Abstract
Urticaria is a common skin condition that, though rarely fatal, can seriously impair a patient’s quality of life. Urticaria is caused by cutaneous mast cell activation and degranulation disease triggered by numerous stimuli. The condition is defined as chronic if it persists for >6 weeks. Self-remission is common in acute urticaria, but in chronic cases less than half of patients achieve remission within 1 year. Diagnosis is typically reached using the patient’s history along with a physical examination. Laboratory workup is based on clinical suspicion and is used to exclude underlying causes, although most cases constitute unknown or spontaneous causes. Extensive routine testing for an exogenous cause is not necessary and does not change the management. This review details the pathophysiology, aetiology, diagnosis, investigation, prognosis, differential diagnosis, and assessment of disease severity, highlighting the potential diagnosis of urticaria and enabling clinicians to make informed assessment decisions.
INTRODUCTION
Urticaria, or hives, is a skin lesion generally described as the pruritic ‘wheal-and-flare’ reaction.1 The wheal is a localised intracutaneous oedema surrounded by a flare, an area of redness or erythema produced as a result of dilated blood vessels. Individual hives can last from 30 minutes to 36 hours and vary in diameter from 1 mm to 20 cm. The central pallor of the hives occurs because the dilated blood vessels in the wheal are compressed. The pathology that characterises urticaria is present in the superficial dermis and includes regional alterations to the venular plexus. The prevalence of urticaria varies according to the population under investigation and the sampling method used. According to a German study, up to 20% of the population will experience an episode of urticaria at some point in their lifetime.2 Overall lifetime prevalence rates of urticaria have been reported as 8.8% of the population.2,3 At any given time, chronic urticaria affects up to 1% of the general population. Both children and adults can acquire urticaria but it appears to be more common among adults, with women affected more often than men. The average age of patients suggests that the condition typically begins in the third to fifth decade of life. No reliable evidence is available regarding the difference in prevalence between race or ethnic groups.2
PATHOPHYSIOLOGY
The wheal-and-flare reaction, which is characteristic of urticaria, occurs as a result of the effects of mediators released from mast cell granules, primarily histamine. Only two of the four types of histamine receptors, H1 and H2, are involved in urticaria. Activation of H1 receptors in the skin induces itching, flaring, erythema, and whealing, whereas activation of H2 receptors contributes only to erythema and whealing.4
The mechanism that stimulates mast cell degranulation is key to the pathophysiology of urticaria. Mast cells express a number of surface receptors, which, upon binding to various molecules, are able to initiate a signal to trigger degranulation; the stimuli that triggers degranulation may be exogenous or endogenous. Cross-linking of mast cell receptors bound to specific immunoglobulin (Ig)E by exogenous allergens, also known as a Type I hypersensitivity reaction, may be relevant to acute urticaria but may not be relevant to chronic spontaneous disease. Other possible exogenous stimuli are pathogen-associated molecular patterns on microbes, which are able to bind to toll-like receptors on mast cells and trigger degranulation.5 This is more often linked to acute viral or bacterial infections than other types of pathogens. Nonimmunologic stimuli include certain drugs, for example opiates, aspirin, and other nonsteroidal anti-inflammatory drugs; neuromuscular blocking agents, such as atracurium; antibiotics, such as polymyxin and vancomycin; and iodinated radiocontrast dyes. In addition, other stimuli, including complements, such as C5a anaphylatoxin, stem cell factor, and some neuropeptides, including substance P, can also trigger mast cell degranulation by binding to their respective receptors available on mast cell surface, independent of the involvement of IgE or IgE receptors.6 Some foods, such as strawberries, also contain histamine-releasing substances. Moreover, direct exogenous histamine intake might occur following consumption of a number of food types, such as cheese, underprocessed scombroid fish, processed meat, tomatoes, pineapple, and avocados.7 Endogenous stimuli that can trigger mast cell degranulation include immune complexes, such as autoantibodies and some autoantigens, for example vasculitis or immune complex from anti-IgE autoantibody, autoantibodies against mast cell IgE receptors (anti-FcεRI-autoantibody), and psychological stress.8,9
Mast cells, which are hypersensitive to physical stimulation, and IgE have been implicated in the pathogenesis of dermographism, cold urticaria, and solar urticaria, without certain mechanisms. In these conditions, physical stimuli induce a neoantigen, specific to IgE antibody, that binds to mast cells. Release of neuropeptides may also initiate or potentiate mast cell activation. Localised platelet clumping has been demonstrated in cold urticaria and, as such, platelet mediators, for example platelet-activating factor and factor IV, may be involved in disease pathogenesis.10 Cholinergic urticaria, another type of physical urticaria, occurs in response to cholinergic sympathetic stimulation. The mechanism causing mast cell activation and histamine release involving cholinergic nerve endings remains unknown.11 For delayed-pressure urticaria, pressure-induced wheals may result from a late-phase reaction, but the initial antigen is still yet to be identified.12 Heat or pressure may cause autoantibody-containing plasma to leak into the extravascular tissue, leading to mast cell activation and degranulation. A non-autoantibody mechanism may also be involved because functional autoantibodies have been detected in approximately 60% of chronic urticaria sera.13 Activation of an extrinsic coagulation cascade was found in chronic urticaria. Increased plasma levels of prothrombin fragments 1 and 2 and D-dimer, a measure of fibrinolysis, have been demonstrated and relate to disease severity, but the contribution of coagulation abnormalities to the pathogenesis of the disease remains unclear.14
Basophils from half of patients with chronic urticaria have been shown to be hyporesponsive to ex vivo anti-IgE stimulation and appear to be associated with basopenia.15 This basophil hyporesponsiveness is caused by elevated levels of the IgE-receptor regulating inhibitory phosphatases, SH2 domain-containing inositol-5-phosphatase 1 and 2, which limits phosphorylation reactions critical for histamine secretion.15 This abnormality appears to reverse with disease remission; therefore, it should be considered a marker of disease activity.15
CLASSIFICATION OF URTICARIA ON THE BASIS OF DURATION
Urticaria is classified as either acute or chronic.1 Acute urticaria is defined by a symptom duration of <6 weeks. Chronic urticaria is generally defined by the presence of urticaria on >3 days of the week, for a period of 6 weeks or longer.
AETIOLOGY OF URTICARIA
The potential causes of new onset urticaria vary, although no specific aetiology can be identified among many patients. Acute urticaria is more likely to have an identifiable aetiology compared with chronic urticaria. Different aetiologies can activate mast cells through many different mechanisms, which are described hereafter.
Infections
Viral, bacterial, and parasitic infections are associated with new onset urticaria. Immune activation, involving immune complex formation and/or complement activation, is one proposed mechanism of urticaria development. Common bacterial infections, such as urinary tract infections, dental infections, Helicobacter pylori, and Mycoplasma pneumonia have also been attributed to the onset of urticaria. Viral illnesses, the most common being picornavirus, as well as coronavirus, respiratory syncytial virus, hepatitis B or C, and HIV infection, have been reported among urticaria patients. Parasitic infections, such as Ancylostoma, Strongyloides, Schistosoma mansoni, Anisakis simplex, and Blastocystis hominis, have been associated with urticaria.
Allergic Reactions
IgE-mediated reactions are associated with urticaria. Antibiotics most frequently implicated in causing IgE-mediated urticaria include beta-lactams, such as penicillins and cephalosporins. Stinging and biting by insects, for example bees, wasps, hornets, fire ants, and kissing bugs, are associated with acute urticaria and are sometimes linked to anaphylaxis. Latex allergy, stimulated by inflation of balloons and use of latex gloves, has also been associated with urticaria. Allergy to foods and food additives is also associated with generalised urticaria, with milk, eggs, peanuts, tree nuts, soybeans, and wheat being the most common foods associated with the condition in children. In adults, fish, shellfish, tree nuts, and peanuts are most often implicated.
Blood product-related urticaria may arise from several mechanisms, including IgE-mediated allergic reactions, complement-mediated reactions, and other immunologic events.
Pseudoallergens
Pseudoallergens include artificial preservatives and dyes in modern processed foods, and aromatic compounds in some natural foods. One in three patients with chronic urticaria undergo a beneficial pseudoallergen-free diet for 3 weeks.16
Mast Cell Degranulation
Drugs, foods, and plants can cause urticaria due to mast cell degranulation through a non-IgE-mediated mechanism. The most frequently implicated substances are narcotics, muscle relaxants, vancomycin, and radiocontrast agents. Opiate analgesics, such as morphine and codeine, cause direct mast cell activation; additionally, opiate derivative ingredients in cough suppressant syrups can also cause urticaria. Moreover, anaesthetic muscle relaxants, including atracurium, vecuronium, succinylcholine, and curare, may cause urticaria. Nonsteroidal anti-inflammatory drugs, such as aspirin, ibuprofen, and naproxen, have been identified as common causes of urticaria.
Physical Factors
Physical urticarial syndromes are forms of chronic urticaria that are triggered by a wide range of specific physical factors. Aquagenic urticaria wheals generally affect the upper part of the body and are induced by water. Cold-induced urticaria is characterised by localised or diffused urticaria that can be accompanied by angioedema within minutes after exposure to a cold object, air, or liquid. Cold urticaria is usually idiopathic, but may occur among patients with cold-dependent antibodies, such as cryoglobulins or cold agglutinins. Delayed pressure urticaria is characterised by wheals or angioedema that develop 4–6 hours after applying any type of pressure, such as wearing tight clothing, hammering, walking, or sitting down. Dermatographism presents as wheals at sites of trauma, friction with clothing, or scratching. Heat contact urticaria is diagnosed when wheals develop 10 minutes after contact with a heat source. Solar urticaria is a less common form of urticaria and occurs following exposure to natural or artificial light sources. Vibratory urticaria is defined by the presence of skin swellings and itching after exposure to vibration at the contact site. Cholinergic urticaria is triggered by elevated core body temperatures, such as during a warm bath, prolonged exercise, or episodes of fever. Exercise-induced urticaria causes wheals during or after exercise.
Causal Agents
Exposure to causal agents for contact urticaria can be classified as nonimmunologic, such as benzoic acid, dimethyl sulfoxide, cobalt chloride, trafuril, polyaminopropyl biguanide (found in wet wipes), melon peel, levofloxacin hydrate ophthalmic solution, and cosmetics, or as immunologic, such as latex, raw meat, fish, potato, phenylmercuric propionate, hair dye, manufacturing facilities, and animal dander.17
Menstruation
Urticaria may worsen premenstrually; however, cases of urticaria that occur predominantly or only premenstrually have been attributed to progesterone sensitivity.
Autoimmune Diseases
Systemic lupus erythematosus, rheumatoid arthritis, Sjögren’s syndrome, and coeliac disease are associated with urticaria. Other autoimmune diseases, such as thyroid autoantibodies, specifically thyroid peroxidase antibodies or antimicrosomal antibodies, are more prevalent among patients with chronic urticaria.
Autoinflammatory Syndromes
Acquired autoinflammatory syndromes, including Schnitzler syndrome, systemic-onset juvenile idiopathic arthritis, and adult onset Still’s disease, have been associated with the onset of urticaria, along with hereditary autoinflammatory syndromes, including cryopyrin-associated periodic syndromes such as familial cold autoinflammatory syndromes, Muckle–Wells syndrome, and neonatal-onset multisystem inflammatory disease; more rarely, hyper-IgD syndrome and tumour necrosis factor receptor-associated periodic syndrome.1
Psychological Factors
Psychological factors, including depression and anxiety, appear to play a contributory role in a proportion of patients suffering from urticaria. Flare-ups of urticaria have been shown to occur at times of psychological stress; however, psychological stress alone is unlikely to be the cause of urticaria.
Malignancy
Chronic urticaria is associated with an increased risk of haematological cancer, for example non-Hodgkin’s lymphoma, and non-haematological cancers, such as brain, retroperitoneal, vulva, kidney, and other urinary systems.18
Unknown Cause
As part of the urticaria diagnosis and management guideline in 2013,1 the term chronic idiopathic urticaria changed to chronic spontaneous urticaria (CSU). In the majority of cases, no cause can be found and hence a CSU diagnosis is made. However, a subset of patients with CSU shows evidence of autoantibodies (chronic autoimmune urticaria), including antibodies to the high-affinity IgE receptor (FcεRI) and/or anti-IgE antibodies.19 Autoimmune urticaria is associated with the IgG anti-IgE receptor (FcεRI) in 35–40% of patients and IgG anti-IgE in 5–10% of patients.20 These autoantibodies can activate blood basophils and cutaneous mast cells.
DIAGNOSIS
The goal of a diagnostic measure is to identify the type of urticaria and the underlying cause. Urticaria is usually diagnosed by taking the patient’s history alongside physical examination, with no investigation required to confirm the diagnosis. The first approach is a thorough exploration of the patient’s history, covering a variety of topics: time of onset, weekly frequency, duration of wheals, provoking factors, diurnal variation, occurrence in relation to workday or weekends, character and distribution of wheals, associated angioedema, itchiness or pain of wheals, systemic symptoms, personal and family history regarding urticaria and allergy, psychiatric diseases, gastrointestinal problems, induction by physical agents or exercise, use of drugs, observed correlation to food, relation to the menstrual cycle, type of work, hobbies, stress, quality of life and sleep, previous diagnosis, previous treatment and response to treatment, and previous investigations and results. Knowing the patient’s history helps to exclude major comorbid disorders and physical urticaria. The approach of urticaria is presented in Figure 1.
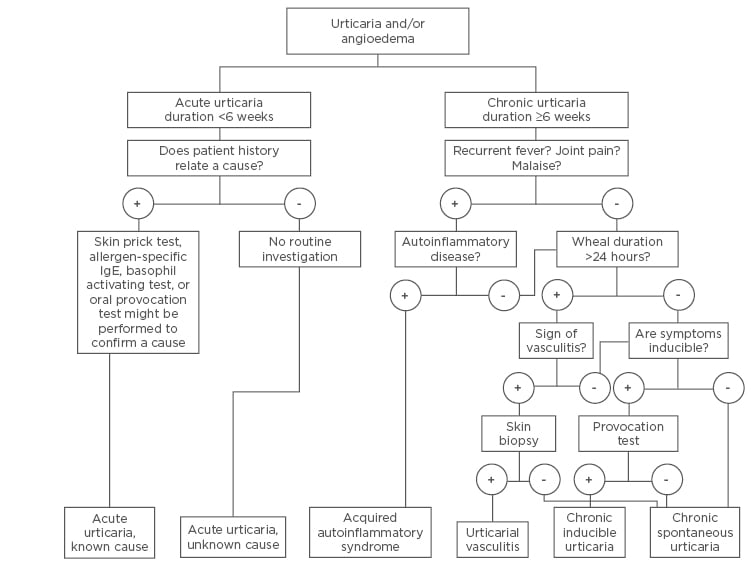
Figure 1: Algorithm for diagnosis and investigation of subtypes of urticaria.
IgE: immunoglobulin E.
The second step of diagnosis is a physical examination. A patient can visit a physician without skin lesions or after the lesions have healed. Skin lesion photographs taken by the patient can aid the diagnosis. The wheal is characterised by central swelling of variable size and the surrounding reflex erythema; the wheals will often dissolve and the skin will return to its normal appearance. Small size wheals (1–3 mm) are usually seen in physical urticaria. Urticarial vasculitis lesions are non-blanching and may be resolved with post-inflammatory hyperpigmentation. Angioedema typically appears as nonpruritic, brawny, nonpitting oedema, with neither well- defined margins nor erythema; swelling usually occurs around the eyes and lips and is also found on the hands, feet, and throat.
INVESTIGATIONS
Following a thorough discussion of the patient’s history and a physical examination, diagnostic provocation tests, including drug, food, and physical tests, are performed as indicated by history and physical examination; moreover, laboratory testing based on related suspicions may also be appropriate. Diagnostic investigation concerning clinical clues and aetiology is summarised in Table 1. If urticaria is suspected to be caused by physical stimuli, a ballpoint pen test for dermatographism, ice cube test for cold urticarial, and pressure tests for delayed pressure urticaria are used to definitively diagnose the disease. IgE-mediated reactions from foods, drugs, or other allergens only rarely result in chronic urticaria; the skin prick test for aeroallergens, food, or serum allergen-specific IgE is used but is not a routine diagnostic test for patients with chronic urticaria.
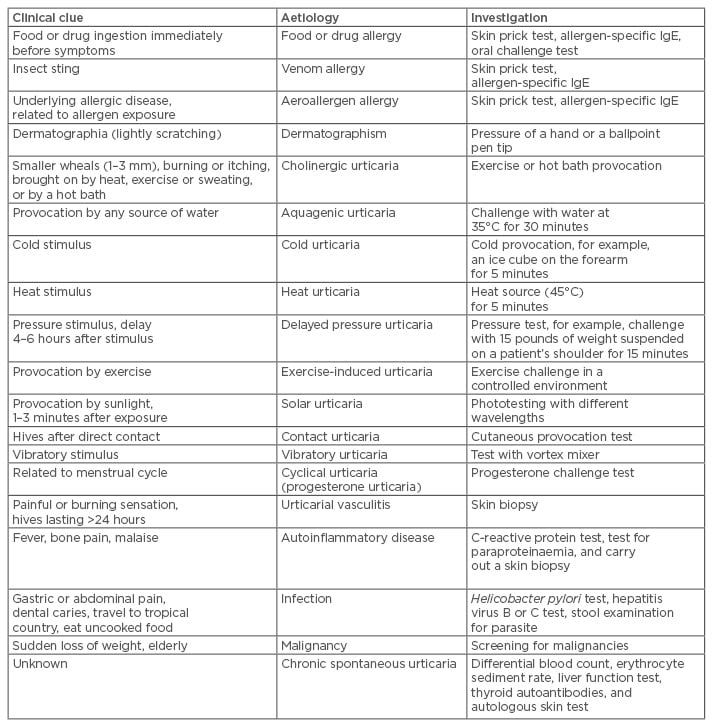
Table 1: Urticaria aetiologies and investigations based on patient history and physical examination.
IgE: immunoglobulin E.
There are a number of patients with CSU showing sensitivity to house dust mites, but those changing disease management remains unclear;21-23 however, most cases of CSU do not have an identifiable cause. Routine laboratory testing can be performed to exclude underlying causes; a high eosinophil blood count can lead to allergy, parasitic disease, autoimmune disease, or tumour. Mildly elevated erythrocyte sedimentation rate can occur in CSU; on the other hand, high erythrocyte sedimentation rate levels are usually linked to urticarial vasculitis and autoimmune disease. Antinuclear antibody is indicated when urticarial vasculitis and autoimmune disease are suspected; however, positive low antinuclear antibody titre (1:80) can detect CSU. Skin biopsy is used when urticarial vasculitis is suspected. Hepatitis B and C titre may be associated with cryoglobulinaemia and some forms of cold-induced urticaria. Thyroid autoantibodies (antithyroid microsomal and peroxidase antibody) are low yield among patients without thyroid-related symptoms. Increased thyroid autoantibodies in titre may be associated with disease duration.24 However, treatment with thyroid hormone among patients with positive autoantibodies presents little evidence to support a low rate of remission.
The autologous serum skin test (ASST) and the autologous plasma skin test (APST) indicating CSU are useful to diagnose autoimmune chronic urticaria. Testing for autoantibodies to anti-IgE and anti-FcεRI is not a routine laboratory measurement. The basophil histamine release test is the gold standard to detect functional autoantibodies, but the diagnosis does not need to be confirmed. ASST or APST are easy to use in practice and demonstrate relevance in vivo to mast cell degranulation and vasopermeability.25 Moreover, ASST and APST can be used to predict remission rate in CSU.26-28 The negative result of ASST and APST before treatment served as a predictor of good prognosis treatment and urticaria remission during a 2-year observational study.26 In addition, negative ASST and negative basophil histamine release test are predictive of fast response to omalizumab;29 oral corticosteroids and antihistamines should be ceased at least 7 days before performing ASST to avoid false-negative results. Serum vitamin D level is not indicated to determine the causes of CSU; however, vitamin D insufficiency is commonly found among patients with CSU and serum vitamin D level is associated with disease severity.30,31 Vitamin D supplements may improve symptoms and quality of life among CSU patients.32-36 Monitoring serum 25-hydroxy vitamin D at baseline is suggested when possible, but is a weak recommendation.
Proteomic technology is used to identify novel autoantigens in chronic urticaria. Testis-specific protein 1 and the macropapain iota subunit of the proteasome multicatalytic endopeptidase complex were identified by proteomic technology as proteins that may be the trigger for urticaria.37 Microarrays and quantitative real-time polymerase chain reaction were used to analyse differentially expressed genes in the phenomenon of wheal development, such as epidermal differentiation, intracellular signal function, transcriptional factors, cell cycle differentiation, inflammation, or coagulation.38
PROGNOSIS
Acute urticaria patients usually develop self-remission within 3 weeks;39 however, approximately 20% of patients with acute urticaria progress to develop chronic conditions.40 The duration of chronic urticaria is typically 1–5 years but may last >5 years in 14% of patients.24 The prognosis of chronic urticaria patients with reported autoimmune urticaria being symptom-free after 1.2 years was 56.5%, while the percentage of CSU patients being symptom-free after 1 year was 34.5%.41 Half of adults with chronic urticaria without expressing angioedema had spontaneous remission within 1 year, whereas 75% of patients with angioedema had persistent disease for >1 year.42 It was also reported that 64.4% of patients with chronic urticaria achieved remission over 2 years.26
DIFFERENTIAL DIAGNOSIS
Other conditions may be confused with urticaria but can be distinguished based on the difference in presentation. Such conditions include urticaria pigmentosa, urticarial vasculitis, atopic dermatitis, contact dermatitis, drug eruptions, erythema multiforme, Henoch–Schonlein purpura, scabies, and viral exanthema. Urticaria pigmentosa presents an orange to brown hyperpigmentation of the lesions and positive Darier’s sign: a wheal-and-flare reaction produced after stroking the lesions.43
ASSESSMENT OF DISEASE ACTIVITY
No specific laboratory test can be used to monitor urticaria activity. Disease activity in CSU can be accessed by an urticaria activity score for 7 days or an urticarial control test.44,45 The urticarial control test can be done by the patient and shows the level of disease activity over the last 4 weeks.
CONCLUSION
Urticaria is a common skin condition and can be diagnosed in the primary care setting. The diagnosis is typically based on noting the patient’s history and performing a physical examination; however, the cause of urticaria is difficult to determine. Investigations should be carried out with a specific test selected based on the patient’s history. As each case differs, extensive laboratory testing is expensive and does not change the prognosis or management. Advances in the understanding of the pathophysiology and causes have helped clinicians to improve diagnosis and management of patients with urticaria.







