Abstract
Introduction: Hyaluronan, or hyaluronic acid (HA), is a naturally occurring glycosaminoglycan present in the skin, joints, and eyes that provides hydration, lubrication, protection, and other important benefits. HA in dermatology is commonly discussed in the context of its anti-ageing properties. However, both pre-clinical and clinical studies have shown numerous applications of HA, low-molecular-weight (LMW) HA, and hybrid LMW/high-molecular-weight HA in dermatology. LMW-HA exhibits antioxidant, anti-tumour, and angiogenic properties, and given its size, an ability to fully penetrate the skin.
Aims: The purpose of this review was to explore the current science and utility of LMW-HA in clinical dermatology and provide an update on its use.
Methods: A PubMed search from 2003–2023 on LMW-HA was conducted to evaluate LMW-HA’s utility in clinical dermatology.
Results: Identified applications of LMW-HA in medical dermatology included treatment of acute and chronic wounds, rosacea, scars, and seborrhoeic dermatitis. Cosmetic applications of LMW and hybrid HA included treatment of skin ageing, enlarged pores, and skin laxity, as well as enhancement of skin hydration. Topical LMW-HA administration promoted healing after cosmetic procedures, chemical peels, and ingrown toenail surgery. Studies of topical and oral LMW-HA demonstrated adequate safety, and newer techniques of administration, such as needleless jet injection, are available.
Conclusion: Exploration and understanding of the properties and benefits of LMW-HA are key to translating its usage in the clinical setting. Basic scientists and dermatologists have achieved substantial progress over the past two decades, and several applications of LMW-HA in dermatology were identified. Additional advantages of LMW-HA are worth exploring.
Key Points
1. This paper provides a comprehensive review of low molecular weight hyaluronic acid (LMW-HA) and its emerging role in dermatology. While hyaluronic acid’s utility is well-established across various medical fields, LMW-HA’s unique properties, such as its antimicrobial effects and ability to penetrate the skin more effectively than its high molecular weight counterpart, present new avenues for clinical applications.2. This scoping review synthesises a wide array of small but significant studies that highlight LMW-HA’s potential in treating conditions such as wound healing, post-procedural care, and ageing, as well as its promise in cosmetic dermatology.
3. By providing an up-to-date exploration of LMW-HA’s dermatologic uses, this paper serves as a foundation for future research and underscores the importance of continued investigation into the safety and efficacy of LMW-HA. As dermatologists seek novel treatments for common and challenging skin conditions, LMW-HA offers an exciting, well-tolerated option that may improve both medical and aesthetic outcomes in certain conditions.
INTRODUCTION
Hyaluronan, or hyaluronic acid (HA), is an extracellular matrix glycosaminoglycan distributed throughout the body, primarily found in the skin, joint synovial fluid, and eyes. It provides key benefits such as hydration and barrier protection. Several medical fields such as orthopaedics, ophthalmology, plastic surgery, periodontics, oncology, pulmonology, neurology, and dermatology have an interest in HA and its potential uses. While the anti-ageing benefits of HA are particularly well known, the applications of HA are quite broad. Countless basic science and clinical studies have explored its utility, ranging from its relevance in the tumour microenvironment to cosmetics and inflammatory conditions. Low-molecular-weight HA (LMW-HA), in particular, is one area of current scientific and clinical interest. This molecule forms from high-molecular-weight HA (HMW-HA) upon hyaluronidase-catalysed depolymerisation and has a number of effects on its local environment.
The appropriate application and utility of HA in dermatology depends on its molecular size, with both HMW and LMW forms providing distinct advantages. HMW-HA provides hydration and barrier function, with size limiting its penetration into the skin.1-2 LMW-HA’s size allows for adequate skin penetration,2,3 and studies have thus explored its potential utility in drug delivery and other cutaneous applications.4,5
There are several applications for crosslinked HMW/LMW-HA, particularly in cosmetic dermatology, and hybrid HA has been utilised in other contexts as well, such as in biomaterials.6
LMW-HA exhibits antioxidant, anti-tumour, and angiogenic properties, and has shown potential benefit in a number of dermatologic and non-dermatologic conditions.7-9 The purpose of this study was to explore the current and potential uses of LMW-HA, specifically in dermatology, as well as avenues for continued research.
METHODS
The authors conducted a PubMed search in January 2024 for articles published on LMW-HA over the past two decades (i.e., 2003– 2023), which yielded 864 articles. Inclusion criteria included studies that were relevant to dermatology; discussed HA, with a focus on LMW-HA or hybrid LMW/HMW-HA; and had at least one of the following: discussion of its application to clinical, medical, or surgical dermatology; molecular properties relevant to clinical practice; or safety. Exclusion criteria included articles outside of the specified date range and non-English articles. Of the 864 articles identified, further evaluation using these criteria elicited 61 relevant articles. A general literature search yielded 14 additional pertinent articles.
RESULTS
Table 1 documents the articles relevant to the current and potential applications of LMW-HA in dermatology, and other contexts of research.
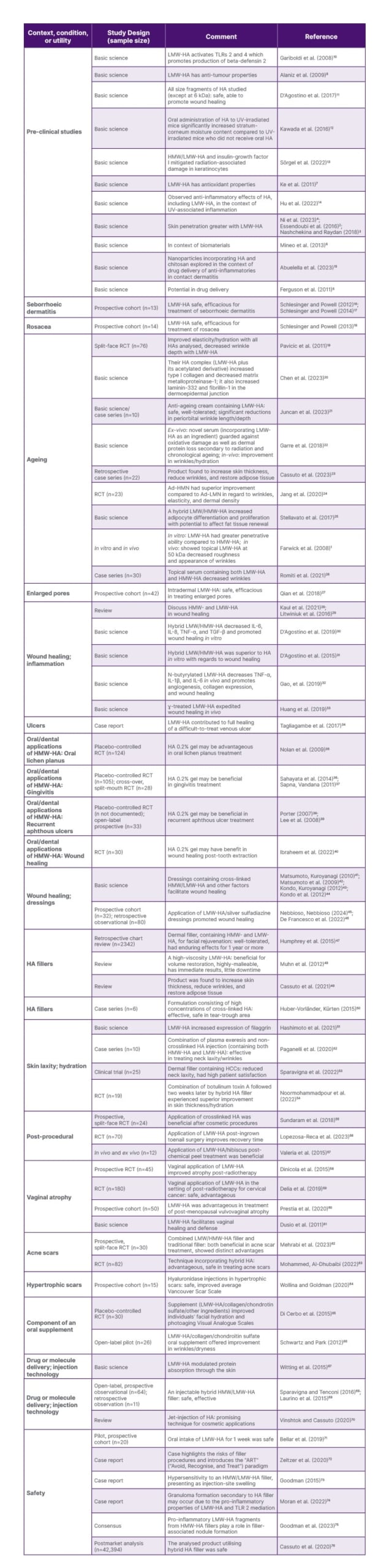
Table 1: Studies of LMW-HA or hybrid/crosslinked HA in dermatology: uses and contexts of research. Dental applications of HMW-HA and pre-clinical studies of LMW-HA are presented as well.
Ad-HMN: adenosine encapsulated-high molecular weight-hyaluronic acid dissolving microneedle patches; Ad-LMN: adenosine encapsulated-low molecular weight-hyaluronic acid dissolving microneedle patches; HA: hyaluronic acid; HCC: hybrid cooperative complex; HMW: high molecular weight; HMW-HA: high molecular weight-hyaluronic acid; LMW-HA: low molecular weight-hyaluronic acid; RCT: randomised controlled trial. TLR: toll-like receptor; UV: ultraviolet.
DISCUSSION
HA is a non-sulfated glycosaminoglycan, consisting of N-acetylglucoasamine and glucuronic acid disaccharides.77 HA has wide medical and cosmetic applications, with effects dependent on molecular size.
The pro-inflammatory nature of LMW-HA is of primary concern, although studies have shown that HAs, even at low molecular weights, are safe to use. LMW-HA ≤20 kDa significantly increased the expression of the pro-inflammatory cytokine, TNF-α, and Farwick et al.1 thus noted that application of LMW-HA ≤20 kDa is not recommended; however, LMW-HA at 50 kDa was safe.1 Furthermore, a study showed that all size fragments of HA studied (except at 6 kDa) were safe and able to promote wound healing, although lower size fragments had higher pro-inflammatory potential.11 While LMW-HA has demonstrated pro-inflammatory properties, Hu et al.14 also observed anti-inflammatory effects of HA, including LMW-HA, in the context of UV-associated inflammation. As introduced, LMW-HA has greater penetrative ability than HMW-HA. Witting et al.67 confirmed this finding and also showed that LMW-HA modulated protein absorption through the skin.67
Studies have examined the utility of LMW-HA in a number of dermatologic applications, including wound healing, seborrhoeic dermatitis, rosacea, scarring, anti-ageing, cosmetics, and post-procedural care. Selected applications are discussed below.
Medical Dermatology
Wound healing
HA has shown utility in several studies on wound healing. LMW-HA activates toll-like receptor (TLR) 2 and TLR4, which promote production of beta-defensin-2, a key molecule in skin microbial defense.10 N-butyrylated LMW-HA decreases TNF-ɑ, IL-1β, and IL-6 in vivo, and promotes angiogenesis, collagen expression, and wound healing.32 Hybrid LMW/HMW-HA decreased IL-6, IL-8, TNF-ɑ, and TGF-β, and promoted wound healing in vitro.30 Additionally, ɣ-treated LMW-HA expedited wound healing in vivo.33 D’Agostino et al.31 showed that hybrid LMW/HMW-HA was superior to HA in vitro with regards to wound healing.
Pre-clinical and clinical studies have evaluated wound dressings incorporating LMW-HA as a means for wound healing. Studies have shown that dressings containing crosslinked HMW/LMW-HA and other factors facilitate wound healing.41-44 A prospective cohort of 32 patients and a retrospective observational study of 80 patients demonstrated that application of LMW-HA/silver sulfadiazine dressings promoted wound healing.45,26 Furthermore, Tagliagambe et al.34 report a case of LMW-HA contributing to full healing of a patient’s difficult-to-treat venous ulcer.
Reviews by Kaul et al.28 and Litwiniuk et al.29 nicely discuss HMW- and LMW-HA in wound healing and the mechanisms/key cells at play in these processes.
Prospective studies showed that LMW-HA was safe and efficacious for the treatment of seborrhoeic dermatitis (n=13) and rosacea (n=14) in these respective cohorts.16-18 LMW-HA has also shown utility in the treatment of acne and hypertrophic scars. A prospective, split-face randomised controlled trial (RCT) of 30 patients showed that combined LMW/HMW-HA filler and traditional filler were both beneficial in acne scar treatment and demonstrated distinct advantages.62 An RCT of 82 patients showed that a technique incorporating hybrid HA was advantageous and safe in treating acne scars.63 Furthermore, a prospective cohort study of 15 patients showed that hyaluronidase injections (prompting LMW-HA formation) in hypertrophic scars were safe and improved the average Vancouver Scar Scale in these patients.64
Cosmetic Dermatology
A prospective study of 42 patients showed that intradermal LMW-HA was safe and efficacious in treating enlarged pores.27
An in vitro study showed that LMW-HA increased expression of filaggrin, a structural protein that contributes to skin hydration and supports the skin barrier.51
Farwick et al.1 showed that, in vitro, LMW-HA had a greater penetrative ability compared to HMW-HA and promoted expression of a greater number of genes compared to HMW-HA. An in vivo extension of their study showed that topical LMW-HA at 50 kDa decreased roughness and the appearance of wrinkles in their small cohort of patients.1
Several studies have described LMW-HA’s effectiveness in skin rejuvenation. A split-face RCT (n=76) showed that LMW-HA led to improved elasticity and hydration of the skin as well as decreased wrinkle depth.19 Chen et al.20 showed that their HA complex (LMW-HA plus its acetylated derivative) increased Type I collagen, decreased matrix metalloproteinase-1, and increased laminin-332 and fibrillin-1 in the dermoepidermal junction.20 Juncan et al.21 (n=10) reported an anti-ageing cream containing LMW-HA that has proven to be safe and well-tolerated. The study found significant reductions in wrinkle length and depth in the periorbital area after 28 days.21 Garre et al.22 analysed ex vivo skin to find that a novel serum, incorporating LMW-HA as one ingredient, guarded against oxidative damage as well as dermal protein loss secondary to radiation and chronological ageing. The study also analysed the skin in vivo, which showed improvement in wrinkles and hydration with the serum.22 A study aiming to analyse the effect of various HA gels on adipocyte-derived stem cells found that a hybrid LMW/HMW-HA increased adipocyte differentiation and proliferation with the potential to affect fat tissue renewal.25 A retrospective case series (n=22) studying a dermal filler containing hybrid HA found that clinically, the gel had high cohesivity upon injection into dermal tissue, and its lower viscosity offered “easy extrusion”.23 The product was found to increase skin thickness, reduce wrinkles, and restore adipose tissue.23 Jang et al.24 compared “adenosine encapsulated” LMW- and HMW-HA “dissolving microneedle patches” (abbreviated Ad-LMN, Ad-HMN). This RCT (n=23) found that both formulations showed skin improvement, but Ad-HMN had superior improvement compared to Ad-LMN in regard to wrinkles, elasticity, and dermal density.24 Finally, a case series (n=30) found that a topical serum containing both LMW-HA and HMW-HA decreased wrinkles, highlighting its anti-ageing effect.26
Regarding LMW-HA’s use in fillers, a retrospective chart review (n=2,342) found that the analysed dermal filler containing HMW- and LMW-HA for facial rejuvenation was well-tolerated, and had enduring effects for 1 year or more.47 Muhn et al.48 conducted an overview of volume restoration using published literature. Of note, they concluded that a high-viscosity LMW-HA is beneficial for volume restoration, and that it is highly-malleable, can be used in different facial planes, has immediate results, and little downtime.48 Another literature review stated that the synergistic effect of LMW-HA and HMW-HA in HA hybrid cooperative complexes (HCC) is ideal for bioremodelling due its ability to increase hydration, elasticity, and skin tone, while supporting the dermis like traditional fillers.49 A case series (n=6) found that a formulation consisting of high concentrations of crosslinked HA was effective and safe in the tear-trough area.50
LMW-HA has also been published in the literature specifically for its use in treating skin laxity. A case series (n=10) found that the combination of plasma exeresis and non-crosslinked HA injection (containing both HMW-HA and LMW-HA) was effective in treating neck laxity and wrinkles.52 A clinical trial (n=25) analysing the HCCs in a relatively new dermal filler found that two injections were effective for reducing neck laxity and resulted in high patient satisfaction.53 A pilot RCT (n=19) evaluated abobotulinum toxin A injection alone versus abobotulinum toxin A injection followed by gel injection of hybrid HA for lower face and neck rejuvenation.54 The study found that patients receiving the combination of botulinum toxin A followed 2 weeks later by the filler experienced superior improvement in skin thickness and hydration.54
Other Low-Molecular-Weight Hyaluronic Acid or Hyaluronic Acid Applications
Post-procedural
Post-procedural advantages involving the use of LMW-HA extend to both cosmetic and surgical procedures. A prospective, split-face RCT of 24 patients showed that application of crosslinked HA was beneficial after cosmetic procedures,55 and an RCT of 70 patients showed that application of LMW-HA post-ingrown toenail surgery improved recovery time.56 An in vivo and ex vivo study of 12 patients revealed that application of LMW-HA/hibiscus post-chemical peel treatment was beneficial.57
An in vitro study found that HMW/LMW-HA and insulin-growth factor I mitigated radiation-associated damage in keratinocytes, thus providing a potential therapeutic avenue for dermatitis associated with radiation treatment.13
Gynaecology
A prospective cohort study of 50 patients showed LMW-HA was advantageous in the treatment of post-menopausal vulvovaginal atrophy.60 A prospective RCT of 45 patients demonstrated that vaginal application of LMW-HA improved atrophy post-radiotherapy.58 Similarly, an RCT of 180 patients showed that vaginal application of LMW-HA in the setting of post-radiotherapy for cervical cancer was safe and advantageous.59 Given LMW-HA’s ability to activate TLRs 2 and 4, promoting antimicrobial polypeptide formation, LMW-HA facilitates vaginal healing and defense.61
Dentistry
HMW-HA has shown utility in the treatment of gingivitis, oral lichen planus, and recurrent aphthous ulcers. HA 0.2% gel may have benefit in oral lichen planus treatment according to a placebo-controlled RCT (n=124), as well as wound healing post-tooth extraction per a recent RCT (n=30).35 Both a placebo-controlled RCT (n=105) and a cross-over, split-mouth RCT (n=28) showed that HA 0.2% gel may be beneficial in gingivitis treatment.36,37,40 Furthermore, a placebo-controlled RCT and an open-label prospective study showed that HA 0.2% gel may be beneficial in recurrent aphthous ulcer treatment.37,39
Oral supplementation
Basic science and clinical research have also explored the utility of oral HA in anti-ageing and facial hydration. A pre-clinical study of skin ageing showed that oral administration of HA to UV-irradiated mice significantly increased stratum-corneum moisture content compared to UV-irradiated mice who did not receive oral HA.12 A placebo-controlled RCT (n=30) showed oral intake of a supplement containing LMW-HA (and other ingredients) twice daily for a month improved individuals’ facial hydration and photoageing Visual Analogue Scales.65 Furthermore, oral intake of a supplement containing LMW-HA (in addition to collagen and another glycosaminoglycan [chondroitin sulfate]) twice daily for three months offered improvement in wrinkles and dryness in an open-label pilot study (n=26).66
Novel Applications
Jet-injection
An open-label, prospective observational (n=64) study and a retrospective observational (n=11) study both showed that a relatively new injectable hybrid HMW/LMW-HA filler was safe and effective.68,69 Jet-injection of HA is a promising technique for cosmetic applications.70
Drug delivery
Nanoparticles incorporating HA and chitosan have also been explored in the context of drug delivery of anti-inflammatories in contact dermatitis.15
Safety
Numerous studies have shown that HA, including LMW-HA, is safe and effective for use in patients both topically and orally. A pilot, prospective cohort study of 20 patients showed that oral intake of LMW-HA for 1 week was safe, and consumption did not alter patients’ microbiome or measured labs.71 Post-market analysis (n=42,394) data revealed that the aforementioned new hybrid HA filler was safe to use.76
Importantly, a key safety risk of HA fillers must be noted, namely, vascular occlusion and necrosis associated with improper use. Zeltzer et al.72 document a patient who experienced facial necrosis from HA filler and received rescue hyaluronidase. This case highlights the risks of filler procedures and introduces the “Avoid, Recognise, and Treat” (ART) paradigm.72 Goodman et al.73 documented a case report of a patient who experienced hypersensitivity to an HMW/LMW-HA filler, presenting as injection-site swelling (ultimately clearing without treatment).73 Granuloma formation secondary to HA filler may occur due to the pro-inflammatory properties of LMW-HA and TLR2 mediation.74 There is consensus that pro-inflammatory LMW-HA fragments from HMW-HA fillers play a role in filler-associated nodule formation.75
Study Limitations
Most studies identified in this scoping review had small sample sizes, limiting external validity. Additionally, publication bias may have skewed the authors’ results in favour of LMW-HA. Furthermore, the articles included had varying methodologies with diverse patient populations, contributing to heterogeneity. This heterogeneity may additionally limit the generalisability of the authors’ study findings. PubMed was the primary search engine for this study, and additional relevant, though non-PubMed-indexed, studies may have been missed. While there are several rigorous basic science studies on LMW-HA, this review focused primarily on the clinical applications of LMW-HA.
CONCLUSION
LMW-HA has received increased attention over the past two decades, and has shown utility and application in several dermatologic conditions, including wound healing, post-procedural healing (after both surgical and cosmetic procedures), ageing, hydration, enlarged pores, facial volume, scars, rosacea, and seborrhoeic dermatitis. New advances have improved drug delivery and effectiveness, and oral supplements containing LMW-HA are available. The safety profile of topical and oral administration of LMW-HA is favourable. Though not a focus of this review, basic science research shows promise for translational applications of HA in clinical practice, and the clinical community will likely see several more advances in subsequent years. Furthermore, the benefits of HA and LMW-HA in other fields, such as dentistry (e.g., HA for recurrent aphthous ulcers, gingivitis, and oral lichen planus) and gynaecology (e.g., LMW-HA for vaginal atrophy), may extend to atrophic, ulcerative, or inflammatory dermatologic conditions. Additional large-scale, well-designed research studies are needed to fully elicit LMW-HA’s potential in the field of dermatology.






