INTRODUCTION
Hair loss is a common problem in clinical dermatology, with many aetiologies including but not limited to androgenetic alopecia, telogen effluvium, alopecia areata, and cicatricial alopecias.1 Identifying effective hair loss treatments is important due to the prevalence of hair loss as well as its impact on quality of life.2 In recent years, new and innovative treatments have emerged to treat hair loss caused by a variety of conditions. This feature article summarises recent advances in the drugs, procedures, and devices used to treat hair loss, summarised in Table 1.
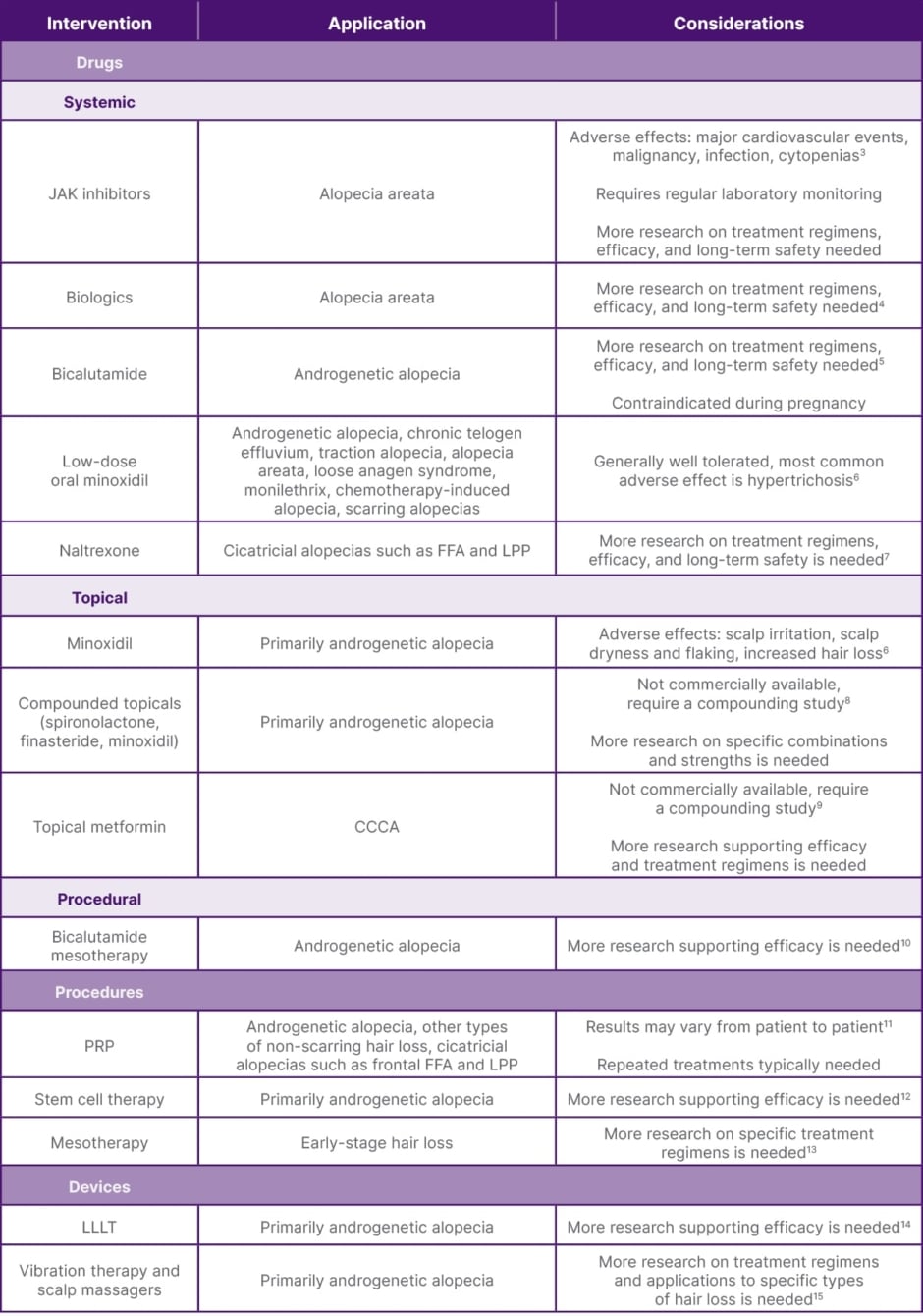
Table 1: Recent advances in the drugs, procedures, and devices used to treat hair loss.
CCCA: central centrifugal cicatricial alopecia; FFA: frontal fibrosing alopecia; JAK: janus kinase; LPP: lichen planoplilaris; LLLT: low-level laser therapy; PRP: platelet-rich plasma.
DRUGS
Oral and topical JAK inhibitors have emerged as promising treatments for autoimmune-related hair loss, particularly alopecia areata.3 JAK inhibitors function through inhibition of enzymes in the JAK/signal transducer and activator of transcription (STAT) signalling pathway, regulating immune system activity. Currently, three JAK inhibitors have been approved by the FDA for the treatment of alopecia areata, including baricitinib,16 ritlecitinib,17 and deuroxolitinib.18 Ruxolitinib is also frequently used off-label to treat alopecia areata.3 Baricitinib is typically started at 2 mg once daily, with dose adjustments based on response and tolerability;19 ritlectinib is typically started at 50 mg once daily;20,21 and deuroxolitinib is typically started at 8 mg twice daily.22 Ruxolitinib is typically started at 5 mg twice daily, with dose adjustments based on response and tolerability.23 Regular lab monitoring is required, including baseline and periodic complete blood counts, liver function tests, and lipid panels, due to potential side effects such as cytopenias, hepatotoxicity, and dyslipidemia. Contraindications include active serious infections, severe hepatic impairment, and a history of thrombosis or malignancy. Although less widely studied than oral formations, topical JAK inhibitors have also shown promise in the treatment of immune-mediated alopecia.11
In addition to JAK inhibitors, biologics targeting IL pathways such as IL-17A, IL-4Rα, IL-13, and IL-12/IL-23p40 are being investigated for their potential roles in treating autoimmune-mediate hair loss such as alopecia areata. IL-12/IL-23p40 inhibitor ustekinumab and IL-4Rα and IL-13 inhibitor dupilumab have both demonstrated efficacy in improving alopecia areata severity in small clinical trials, while IL-17A inhibitor secukinumab did not demonstrate clinical efficacy in reducing alopecia areata severity in a small trial. Further prospective studies with larger cohorts are needed to determine the efficacy and optimal dosing regimens of biologics for treating alopecia areata and other alopecias.4
Although topical minoxidil has frequently been used to treat a variety of conditions causing hair loss, low-dose oral minoxidil has emerged as a treatment for a variety of alopecias in recent years. Studies have demonstrated low-dose oral minoxidil to be generally well tolerated, with the most common adverse effect being hypertrichosis. Although most commonly studied as a treatment for androgenetic alopecia, low-dose oral minoxidil has also demonstrated efficacy for treating chronic telogen effluvium, traction alopecia, alopecia areata, loose anagen syndrome, monilethrix, chemotherapy-induced alopecia, and scarring alopecias.6
Compounding of systemic medications into topical formations has also gained traction outside of JAK inhibitors, allowing for decreased systemic absorption and associated side effects. Medications such as spironolactone, which blocks the effects of androgens, and finasteride, which inhibits the conversion of testosterone to dihydrotestosterone, have been formulated into topical versions to treat androgenetic alopecia, with studies demonstrating efficacy in promoting hair regrowth while avoiding hormonal side effects. Topical combinatorial treatments that include minoxidil have also been demonstrated to improve hair growth in the treatment of many forms of alopecia. For example, topical minoxidil combined with topical spironolactone was found to be superior to topical minoxidil alone. Further research on the most effective combinations and strengths is needed.8
Repurposing of drugs initially intended to treat other conditions has also emerged as a potential treatment for alopecia. Topical metformin, an insulin-sensitising drug typically used orally to manage diabetes, has demonstrated potential anti-fibrotic and anti-inflammatory effects beneficial for promoting hair regrowth in patients with central centrifugal cicatricial alopecia. Although this is still an emerging area of research, initial studies have suggested that topical metformin can decrease inflammation and fibrosis associated with central centrifugal cicatricial alopecia, offering a new route for treatment.9 Bicalutamide, an antiandrogen used in the treatment of prostate cancer, has demonstrated efficacy in treating female androgenetic alopecia in retrospective studies, particularly when used in combination with oral hormonal contraceptives. Prospective studies are needed to determine efficacy and optimal dosing regimens.5 Low-dose naltrexone, an opioid receptor antagonist that has demonstrated anti-inflammatory and analgesic effects, has shown efficacy in treating scalp pruritus and erythema in primary cicatricial alopecias including lichen planopilaris and frontal fibrosing alopecia. Due to the retrospective nature of existing research, further prospective studies are required to determine efficacy and optimal treatment regimens.7
PROCEDURES
Platelet-Rich Plasma (PRP) therapy is increasingly used to treat androgenetic alopecia and other types of non-scarring hair loss.24 PRP is derived from the patient’s own blood, which is processed to concentrate platelets and injected back into the scalp with the belief that the growth factors in the PRP may stimulate hair follicles and promote hair regrowth. Common dosing regimens for PRP therapy in alopecia typically consist of injections every 4 weeks for three sessions, followed by maintenance therapy with one to three rounds every 6–12 months.25 This approach helps maintain results over time; however, studies of PRP efficacy have been limited by small sample sizes and inadequate follow-up times.26 Although PRP therapy has been widely used with studies supporting its effectiveness in improving hair density and thickness, the exact mechanism behind its efficacy is not fully understood and results may vary from patient to patient.
Stem cell therapy represents a cutting-edge therapy for the treatment of hair loss, particularly androgenetic alopecia and forms of non-scarring hair loss.12 This treatment involves harvesting, processing, and injecting the patient’s own adipose-derived,27 mesenchymal,28 or hair follicle-derived stem cells into the scalp. Stem cells are believed to modulate androgenic alopecia by regenerating hair follicles and enhancing hair growth.12 Stem cell therapy is an emerging treatment for hair loss, and research regarding its long-term efficacy and safety is ongoing.
Mesotherapy, which involves injecting a solution of medications such as minoxidil, growth factors, vitamins, stem cells, or botulinum toxin A into the scalp, is used to treat early-stage hair loss and improve overall hair health. This delivery approach allows for product to more effectively reach hair follicles, possibly increasing the longevity and effectiveness of injected treatments as compared with topical preparations. Common dosing regimens for mesotherapy vary by treatment type, but typically consist of weekly treatments for 7–8 sessions, followed by biweekly treatments for 8–9 sessions, followed by monthly treatments for 3–12 sessions. Dutasteride mesotherapy, for example, has demonstrated the most efficacy when dosed weekly for seven sessions, then biweekly for nine sessions, followed by monthly for 12 sessions. Although studies have reported improvements in hair growth after treatment, there is no standardised treatment regimen and research is still in the early stages.13 Bicalutamide mesotherapy has recently emerged as a potential treatment for female androgenetic alopecia, but further studies are required to determine treatment efficacy.10
DEVICES
In addition to pharmacological treatments and procedures, several at-home devices have gained popularity for promoting hair growth. These devices offer patients the convenience of self-administration and may become more cost-effective than in-office treatments over time.
Low-level laser therapy (LLLT) uses low-energy lasers with wavelengths between 600–1,100 nm to stimulate hair follicles and improve hair growth.8,29 The lasers penetrate the scalp, enhancing cellular metabolism and blood flow to the hair follicles, which is believed to increase hair density. This treatment has demonstrated efficacy for treating androgenetic alopecia and other non-scarring alopecias; however, there is a scarcity of controlled studies to support the efficacy of LLLT.14 Red light at 650 nm30 or near-infrared light at 600–950 nm29 is believed to stimulate the mitochondria of the hair follicles, enhancing energy production and stimulating hair regeneration. Research supporting the efficacy of LLLT is limited, and further studies are necessary to standardise treatment.
Vibration therapy and scalp massagers are designed to promote scalp blood flow and hair growth via mechanical stimulation and relaxation of the hair follicles.15 The underlying mechanism is believed to be caused by mechanical stimulation affecting the cells and genes involved with hair growth, leading to increased hair thickness. Further studies to determine treatment regimens and applications for specific types of hair loss are needed to further understand this emerging method for hair regrowth.
CONCLUSION
Recent advances in hair loss treatments reflect a growing array of options for managing various causes of hair loss. Medications, procedures, and medical devices have all emerged as potential therapeutic avenues, and continued research and clinical trials will be essential to validate the efficacy and refine the applications of these treatments.






