Overview
Psoriasis is a chronic inflammatory disease that is associated with a reduction in quality of life (QoL) and professional work productivity.1-3 Clinical data presented at the 26th European Academy of Dermatology and Venereology (EADV) congress regarding certolizumab pegol (CZP), an Fc-free pegylated anti-tumour necrosis factor (TNF) antibody, demonstrated a clinically meaningful and sustained psoriasis treatment effect associated with maintained improvements in QoL and work productivity.4-7
Patient-Specific Data
Young women affected by psoriasis require safe and effective disease treatments. Control of disease before, during, and after pregnancy may contribute to the prevention of adverse pregnancy outcomes and reduce the risk of post-partum flares. Women who experience worsening of psoriasis during or after pregnancy may require personalised treatment.8 Anti-TNF treatments are effective therapies that are often stopped during pregnancy and breastfeeding due to limited evidence in this population.
Because of its molecular structure, CZP is not thought to be actively transported across the placenta.9 The CRIB study used a highly sensitive CZP-specific assay to analyse the level of placental CZP transfer in 14 mother-infant pairs treated with commercial CZP on either CZP 200 mg every 2 weeks (Q2W) or CZP 400 mg every 4 weeks (Q4W), for a locally approved indication (rheumatoid arthritis, axial spondyloarthritis/ankylosing spondylitis, psoriatic arthritis, and Crohn’s disease). Mother, baby, and cord blood samples were collected at birth; the baby’s blood was further sampled (4 and 8 weeks post-partum). Infant CZP levels were either minimal or below the limit of detection in all blood samples at all time points. At birth, 13 of 14 infants had no quantifiable CZP levels (lower limit of quantification: <0.032 μg/mL) and 1 infant had a minimal CZP level of 0.042 μg/mL (infant/mother plasma ratio: 0.0009) (Figure 1). No infants had quantifiable CZP levels at Weeks 4 and 8 post-partum. The generated evidence indicated no-to-minimal in utero fetal CZP exposure, supporting continuation of CZP during the third trimester of pregnancy if required.10
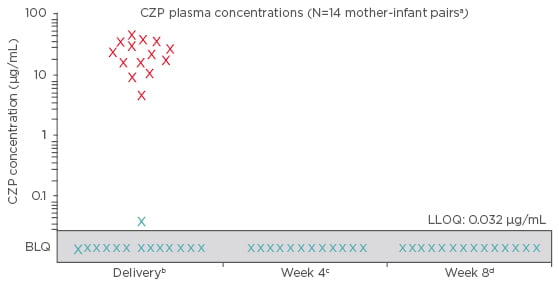
Figure 1: Certolizumab pegol concentrations for both mothers and infants during the CRIB trial.
X: maternal concentrations (collected within 24 hours pre or post-delivery).
X: infant concentrations (collected within 24 hours post-delivery).
a2 of 16 infants were excluded from the per protocol analysis set, 1 due to missing data at birth and 1 due to implausible pharmacokinetic data at birth (i.e. data not consistent with a paediatric CZP pharmacokinetic model based on the expected range of clearance, volume of distribution, and subsequent elimination half-life); b ±24 hours; c ±7 days (two samples not collected); d ±7 days.
BLQ: below the limit of quantification (<0.032 μg/mL); CZP: certolizumab pegol; LLOQ: lower limit
of quantification.
Post-partum flares commonly occur in women with rheumatic diseases and in >50% of patients with psoriasis, causing a treatment dilemma.8,11,12 The CRADLE study used the same CZP-specific assay to analyse the CZP concentration in 137 breast milk samples from 17 breastfeeding mothers requiring post-partum CZP treatment (CZP 200 mg Q2W or CZP 400 mg Q4W).13 Approximately half the samples, 77 of 137 (56%), had no measurable CZP, 52 (38%) had minute but measurable CZP levels (<0.064 μg/mL), and 8 (6%) had low CZP levels (<0.096 μg/mL). The highest measured CZP concentration in breast milk (0.0758 μg/mL) was <1% of the expected plasma trough concentration the adverse effects in mothers were consistent of a therapeutic dose and the median CZP relative with the established safety profile of CZP, infant dose (0.15%) was considered within the safe and those in infants were consistent with those in unexposed infants of a similar age.







