Abstract
Some types of chronic urticaria (CU) are associated with autoreactive immunoglobulin (Ig)E, as well as IgG. In the syndrome of autoimmune thyroid disease and CU, autoreactive IgE, as well as IgG against host thyroid tissue, is present. The author describes a patient with new onset of CU after vasectomy with evidence of both autoreactive IgE and IgG anti-sperm antibodies (ASA). Autoreactive sperm proteins are enzymes opposed to structural sperm antigens producing ASA in infertility and after anti-spermatocyte vaccines. The author suggests that autoreactive proteins with enzymatic activity either in host proteins, aeroallergens, or viral proteins may have increased propensity to generate autoreactive IgE. This model of autoimmune IgE ASA generation by sperm and other host enzymatic proteins in CU can be tested using proteomic technology.
INTRODUCTION
Chronic urticaria (CU) is a syndrome in which symptoms persist for >6–8 weeks in the absence of an identified trigger.1,2 Genetic and proteomic data, if available, as well as response to pharmacologic agents, are useful information that should be used to diagnose CU.3 For example, response to targeted therapy, such as omalizumab, may provide unexpected insights into CU pathogenesis.4-7 A response to omalizumab implicates immunoglobulin (Ig) E and the IgE receptor in many forms of CU. While the terms ‘idiopathic’ or ‘spontaneous’ are often used to describe CU, the author and others have suggested that these terms do not contribute any information to the diagnosis and should be eliminated.
In many cases CU is associated with autoimmune disease and autoreactive Ig, as shown by the ability of CU serum to activate donor basophils.8-10 However, there is uncertainty regarding which proteins may serve as autoantigens in CU.11 Regarding the autoreactive proteins that might serve as targets in proteomic studies and the mechanisms responsible, IgG against the IgE receptor and IgE have been described in many patients with CU. However, these IgG autoantibodies are not always present and do not correlate with disease severity or progression.12 T lymphocyte proliferation has also been reported in response to the host IgE receptor protein in patients with CU although, as with autoreactive IgG, the prognostic significance of T cell proliferation in prospective studies of CU remains to be established.8
Another possible mechanism of CU is that autoreactive IgE, rather than autoreactive IgG, is responsible for priming mast cells to respond to host antigens. For example, patients with autoimmune thyroid disease are more likely to develop CU if they have autoreactive IgE, as well as IgG against thyroid tissue.13,14 Autoreactive IgE could explain the intermittent nature of symptoms in CU, since mast cell activation would occur only with the combination of autoreactive IgE and flares of thyroiditis or other forms of tissue damage, such as viral replication in thyroid tissues releasing thyroid antigens into the circulation.15
In support of the autoreactive IgE hypothesis in CU, the author describes an interesting case of urticaria after vasectomy in which proteomic analysis with technology developed for studies of anti-sperm antibodies (ASA) was used to determine the presence of atypical sperm antigens after vasectomy and in infertility syndromes.16-19 Inflammation and autoimmunity after vasectomy is common but usually self-limiting.20,21 Similarly to antithyroid IgE, anti-sperm IgE, seminal fluid IgE, and IgG can occasionally persist due to a combination of human leukocyte antigen (HLA) presentation of antigens and inflammatory cytokines, arming mast cells to self-antigen as suggested by previous clinical reports.22-25 If confirmed by additional proteomic studies, these observations could be useful in suggesting why some patients may generate autoreactive IgE and IgG against host tissues and viral proteins associated with urticaria.
CLINICAL PRESENTATION OF A PATIENT WITH NEW ONSET AUTOIMMUNE URTICARIA AFTER VASECTOMY
A previously healthy 39-year-old man presented with daily severe CU after an uneventful vasectomy in June 2002. The patient was previously fertile and had obtained a vasectomy for contraception. Urticaria started about 2 weeks after the vasectomy and continued every day with no specific triggers identified. The urticaria proved resistant to therapy with non-sedating antihistamines, oral corticosteroids, and cyclosporine A and levothyroxine. He had no urticaria or allergy previously. Skin and radioallergosorbent testing was negative to common aeroallergens and foods. He reported a flu-like illness immediately after the vasectomy that resolved after several days. Serum was obtained in 2003 after written informed consent for experimental diagnostic studies.
Due to the unusual presentation of the urticaria associated with a surgical procedure, some additional studies were obtained to clarify the diagnosis and assess whether an underlying systemic illness or infection was present. Stool for ova and parasites was negative, and serum total IgE was slightly elevated at 166 international units (IU)/mL (nl<114). Additional laboratory evaluation showed an anti-nuclear antibodies (ANA) positive 1:40 speckled pattern anti-DNA negative. Anti-thyroglobulin (7 IU/mL, nl<2) and anti-microsomal thyroid peroxidase antibodies (44 IU/mL, nl<2) were present, but thyroid stimulating hormone was normal as were liver function and complete blood count. IgG and IgM serology for hepatitis B and C were negative.
When tests were repeated after >1 year of daily CU, thyroid autoantibodies were present at unchanged levels, while previous borderline-positive ANA testing was no longer positive. A serum CD203c basophil activation assay demonstrated one of the highest values recorded in this assay, with approximately 50% activation of donor basophils by the patient’s serum versus ≤5% for patients without CU and a mean value of >10% in patients with CU. The patient’s HLA type was HLA B8 (BW6), B27 (BW4); HLA B27 is associated with increased risk of pustular psoriasis and other rheumatological conditions.
The basophil activation assay confirmed that the patient’s urticaria was directly related to a circulating autoreactive Ig. However, the relationship between the epitope recognised on mast cells and basophils by the patient’s serum was not established further in this study. Autoantibodies detected against sperm proteins could be identical, with the autoantibodies triggering mast cell and basophil release in the CD203c assay, or alternatively these could be distinct autoreactive antibodies triggered by epitope spreading or related mechanisms (see the ‘Discussion’ section). At the most recent follow-up, more than a decade after the onset of symptoms, the patient continued to have daily urticaria. He had also developed clinically significant thyroid autoimmune disease diagnosed after an episode of clinical depression and responding to replacement therapy with thyroid hormone.
As a result of the positive thyroid autoantibodies and transient positive ANA, as well as HLA type associated with autoimmune skin disease, it seemed possible that the patient’s urticaria resulted from autoreactive Ig triggered by events associated with the vasectomy and possible systemic exposures to autoreactive sperm proteins. Therefore, as described in the ‘Materials and Methods section’, additional studies were performed at no risk or cost to the patient using technology developed for studies of anti-sperm immune response in infertility research to characterise whether anti-sperm auto-IgG or IgE antibodies were present. The rationale for performing studies to identify urticaria associated autoreactive sperm antigens in this patient was that results could be compared to previous results from a large number of serum samples from healthy men after vasectomy and other conditions, such as infertility associated with ASA, which have previously been characterised using methods similar or identical to those described for this patient.18,19 The findings of previous studies mean the normal immune response to vasectomy and other forms of ASA formation in patients without CU is well understood.
MATERIALS AND METHODS
Serum from the patient was obtained after >1 year of daily urticaria and was used to probe a sperm protein two-dimensional (2D) gel representing an array of human sperm proteins. Serum was used fresh within 48 hours and not frozen or otherwise processed, and was not available from the patient prior to the vasectomy. Conditions for the extraction of sperm proteins were optimised to enrich for cell surface expression, because proteins on the sperm surface would be expected to generate an autoimmune response as described.18,19 Samples were focussed in the first dimension using immobilised (11 cm, 3–10 nl) pH gradient strips, and the second dimension was run on 8–16% Criterion gels. Both immobilised pH gradient strips and Criterion gels were obtained from Biorad, Hercules. California, USA.
Sample blots were compared against control blots of 13 pooled ASA negative male sera. None of the control serum donors were known to have CU, systemic illness, or autoimmune disease, and neither serum from other patients with CU were available for analysis in this study, nor have other proteomic studies of anti-sperm autoantibodies in CU been published in peer-reviewed form to the author’s knowledge. Experiments were repeated to give n=3. Silver-stained gels were run on the same day in parallel with gels for blots. Positive spots were checked against controls and only those spots that were not present in control samples were cored from silver-stained gels and sent for tandem mass spectrometry microsequencing. Microsequencing results (Report number: H2D-040524-001) were obtained from the W.M Keck Biomedical Mass Spectrometry Laboratory, University of Virgina, Charlottesville, Virginia, USA.
Membranes containing blotted antigens, previously subjected either to 1D or 2D electrophoresis were incubated (1 hour, at room temperature) in the blocking solution (5% dry milk, 0.05% Tween-20 [vol/vol], phosphate buffered saline, pH 7.4). After washing, the blots were incubated (4°C, overnight) with the serum sample diluted 1:2,000 in the blocking solution. The horseradish peroxidase-conjugated secondary antibody (Donkey Anti-Human IgE, and IgG all subclasses [IgG data not shown]; Jackson ImmunoResearch, West Grove, Pennsylvania, USA) was then applied and the enzyme products were visualised by enhanced chemiluminescence using the manufacturer’s protocol (Amersham, UK).
Levels of autoreactive Ig were not determined in the patient’s tissues or seminal fluid in this study but may be of interest in future studies, and western blotting was preferred over other methods of autoantibody characterisation, such as enzyme-linked immunosorbent assay, to permit direct gel extraction and microsequencing of autoreactive proteins. Sera were not tested against the patient’s own sperm, and as such the immunoreactive proteins are properly referred to as iso or alloantigens; however, for the purpose of this discussion they will be termed ‘autoantibodies’.
IDENTIFICATION OF IgG AND IgE REACTIVE AUTOANTIGENS AS COMPONENTS OF CATALYTICALLY ACTIVE PROTEASOMES EXPRESSED IN SOMATIC HOST TISSUES
Of particular interest in the case of the present patient, was that the dominant immune response was directed to proteins that are components of proteasomes; furthermore, not only sperm-specific IgG, but IgE, was evident. Two approximately 28 kDA sperm proteins were highly reactive with both IgG and IgE in the patient’s serum and were not detected in parallel tests with 13 pooled samples of serum from ASA negative men (IgE stained gels shown in Figure 1, IgG results not shown). Identity of these two proteins was determinedby microsequencing with tandem mass spectrometry of the 28 kDa spots cored from a silver-stained 2D gel that yielded specific peptides (Table 1).

Figure 1: Electrophoretic two-dimensional gels.
A) Silver-stained for total proteins in the left panel, and B) Immunoblot using the patient’s serum with secondary anti-human IgE antibody. Two proteins are evident that bind specifically with IgE from the patient’s serum. These two proteins also bind to IgG from the patient’s serum (IgG data not shown). Identification of these autoreactive proteins from proteomic analysis of peptides derived from extraction of these spots is shown in Table 1 and discussed in more detail in the text. Ig: immunoglobulin.
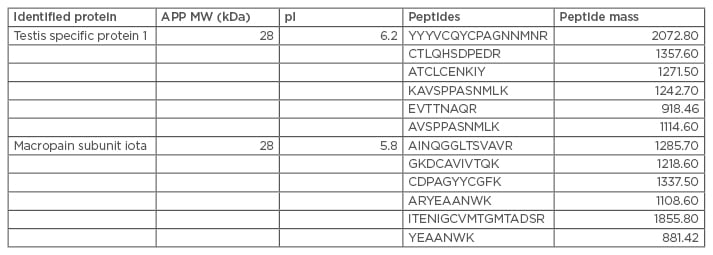
Table 1: Microsequencing with tandem mass spectrometry of 28 kDa spots cored from a silver-stained two-dimensional gel, which yielded specific peptides.
APP: amyloid precursor protein; MW: molecular weight.
A bioinformatic search of the National Center for Biotechnology Information (NCBI) protein databases revealed the proteins to be testis specific protein 1 (NCBI accession number: NP_003287), and similar to the alpha subunit, or the macropain subunit iota of the proteasome multicatalytic endopeptidase complex (NCBI accession numbers: CAA43964, AAH5552). Testis specific protein 1 is a member of the cysteine-rich secretory protein (CRISP) protein family.26 The proteasome multicatalytic endopeptidase complex is a multicatalytic proteinase complex. Searching UniGene for tissue expression specificity information for testis specific protein 1 gave the following complementary DNA (cDNA) sources: testis, medulla, hippocampus, pooled germ cell tumours, prostate, human lung epithelial cells, and pooled metastatic prostate bone lesion, suggesting expression in the brain and lung in addition to the reproductive tract. CRISP-3 has been identified as a defence-associated molecule with predominant expression in the salivary gland, pancreas, and prostate.
The broad pattern of tissue distribution is striking for the alpha subunit, or the macropain iota subunit of the proteasome multicatalytic endopeptidase complex. The proteasome is a multicatalytic proteinase complex that is characterised by its ability to cleave peptides with broad specificity at Arg, Phe, Tyr, Leu, and Glu adjacent to the leaving group at neutral or slightly basic pH. UniGene for tissue expression specificity information for alpha subunit of the proteasome multicatalytic endopeptidase complex yields an array of cDNA sources, including heart, kidney, brain, mixed tissue, chondrosarcoma, cartilage, pituitary, dorsal root ganglia, brain motor neuron, hypothalamus, prostate tissue, lung, spleen, and ovary. No primary sequence similarity was evident between these two proteins by standard sequence alignment algorithms, nor was any primary sequence similarity evident between either of the proteins and thyroid microsomal peroxidase, the thyroid autoantigen most closely linked with autoimmune urticaria.
PROTEASOME-ASSOCIATED IgE AND IgG AUTOREACTIVE SPERM ANTIBODIES ARE DISTINCT FROM AUTOREACTIVE SPERM ANTIBODIES DETECTED IN PATIENTS WITH OTHER FORMS OF ANTI-SPERM ANTIBODIES
The repertoire of immunodominant proteins recognised by infertile men with ASA is usually directed to testis-specific differentiation antigens unique to spermatocytes or spermatids.16-19 Post-meiotic differentiation antigens that comprise the unique cyto-architectural features of the sperm, such as acrosome, outer dense fibres, and fibrous sheath, are frequently recognised. These post-meiotic antigens include the acrosomal proteins SP-10, SAMP14, SAMP32, and equatorial segment protein, and the fibrous sheath proteins AKAP3 (FSP95) and CABYR. Notably, these typical ASA are not associated with proteasomes.
In contrast, testis specific protein 1 is a member of the CRISP family. This protein contains SCP/Tpx-1/Ag5/PR-1/Sc7 family of extracellular domains. These domains are shared by the human glioma pathogenesis-related protein GliPR and a plant pathogenesis-related protein, and represent functional links between plant defence systems and the human immune system. These domains have no known function but appear to be highly antigenic. CRISP-3, from the same family as testis specific protein, plays a role in the pathophysiology of at least two other autoimmune diseases: Sjogren’s syndrome and chronic pancreatitis. Similarly, the second ASA associated with onset of CU in this patient denoted ‘similar to the alpha subunit, or the macropain iota subunit of the proteasome multicatalytic endopeptidase complex’ is a proteasome associated protein not present in the normal ASA response.
DISCUSSION
As suggested in this paper, ASA-generated Ig due to novel antigenic exposures of sperm proteins to the immune system could provide a mechanism of autoimmune urticaria through molecular mimicry between sperm and somatic cell proteins, as well as the related phenomena of epitope spreading from tissue autoantigens to other tissue autoantigens. The autoreactive IgE detected in the patient studied in this paper represents a mechanistic link between autoimmunity and mast cell activation resistant to normal therapy, such as antihistamines and oral corticosteroids. Unfortunately, the patient was not interested in a trial of omalizumab, a humanised monoclonal antibody that specifically blocks IgE mediated mast cell activation; however, omalizumab and related therapy might be useful and informative in patients with this syndrome in the future.
Normally, suppressor cells induced at mucosal surfaces should prevent further progression of the immune response to generate autoreactive IgE or IgG (Figure 2). Current understanding of autoimmune mechanisms suggests that mucosal immune responses mediated by transforming growth factor (TGF)-β and interleukin-10 should suppress autoreactive IgE and IgG responses through the generation of IgA and suppressor T-lymphocytes, respectively. However, in the patient described, these protective mechanisms were apparently not functional, perhaps due to the proposed proteasome-medicated activation of IgE and IgG. In support of this hypothesis, the flu-like symptoms experienced by the patient after the vasectomy, but prior to onset of urticaria, could correspond to the release of T helper cell (Th)1 and Th2 inflammatory cytokines, rather than Th3 suppressive cytokines during primary ASA response to sperm proteins after vasectomy.
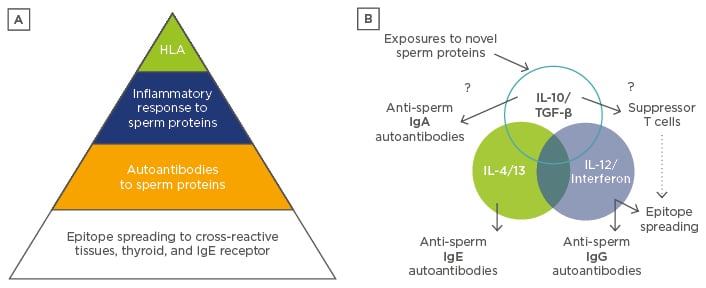
Figure 2: A model of autoimmunity after vasectomy.
A) Regulatory defects, including particular HLA antigens capable of presenting sperm proteins in altered configurations or co-incident inflammatory responses at the time of vasectomy, lead to epitope spreading with cross-reactive IgE and IgG antibodies binding to mast cells. Proteasome antigens contribute to inflammation and TH2 or TH1 cytokines, respectively, in a small minority of men after vasectomy as illustrated. B) TH3 cytokines, such as IL-10 and TGF-β, present on mucosal membranes should normally induce suppressor cells and IgA, which block or prevent further IgG and IgE autoantibody production, unless counteracting inflammatory stimuli, such as sperm proteasome antigens, shared with host tissues dominate the immune response. Strong innate inflammatory signals delivered by sperm or viral proteases could drive IL-4 and IL-13 polarised IgE responses, as discussed in more detail in the text.
HLA: human leukocyte antigen; Ig: immunoglobulin; IL: interleukin; TGF: transforming growth factor; TH: T helper cell.
Based on these observations, the author suggests that this patient and previous reports of CU and/or anaphylaxis after vasectomy represent a putative new syndrome characterised by ASA proteasome-associated (ASAp). Remarkably, both atypical sperm proteins detected in this study are related to other inflammatory proteins. The CRISP proteins are not only related to members of the plant PR-1 proteins and tumour GliPR proteins, as noted above, but are also present in many snake venoms, where they are thought to trigger hypothermia through interference with membrane channel signalling. Human mast cells are highly sensitive to non-specific effects of snake venom proteases, such as hypothermia triggering mast cell release, and depletion of mast cells results in markedly increased mortality from venom.27 These observations suggest that a primary focus of the mast cell and IgE system is co-ordinated with innate immune resistance to proteases, providing increased survival from snake and other venoms.28,29 In direct support of this hypothesis, novel innate mechanisms of mast cell activation by a papain protease has been described in which a protease substrate on the mast cell surface appears to undergo cleavage by papain causing non-IgE mediated mast cell activation and degranulation.30
Activation of the innate immune system by proteases, which are often also encoded by chronic viral pathogens as well as sperm proteins, is possibly a critical initial event in CU, thus accounting for those patients who lack IgE receptor autoantibodies and yet have antithyroid antibodies and CU and angioedema.15 Protease- mediated activation of the innate immune system could, in turn, contribute to other findings in CU and related angioedema, such as complement activation and related innate mechanisms of inflammation.31-33 Further studies of ASA and protease antibodies in CU and related syndromes, such as autoimmune thyroiditis, might help to clarify both the aetiology of these disorders, as well as their markedly increased prevalence in females compared to males.
In summary, an inflammatory response to sperm or viral proteases in this patient could both promote IgE and also break tolerance to other mast cell proteins, such as the mast cell and basophil IgE receptor present at the site of the inflammatory reaction, due to phenomena such as epitope spreading as previously described for IgG- mediated autoimmune disease.34-37 An important prediction of this hypothesis is that both men and women with CU might, in some cases, have evidence of IgE-mediated autoimmunity to sperm proteases described in this work as well as to viral and host proteases.







