Abstract
Introduction: Parry–Romberg syndrome (PRS) is a rare neurocutaneous condition that can affect the skin, subcutaneous fat, muscle, and bone on one side of the face. PRS presents with progressive but self-limiting facial hemiatrophy, and its severity ranges from barely noticeable asymmetry to severe disfigurement. The authors describe a 35-year-old male with right facial hemiatrophy. The patient presented in a stable condition at the initial consultation.
Objectives: Adipose tissue autografts were the most used tool to treat many congenital or acquired facial deformities. The authors proposed using cross-linked hyaluronic acid (HA) fillers over those traditionally used to correct facial deformities due to PRS.
Discussion: PRS is usually self-limiting, with the maximal progression of the disease 2–5 years after onset. There is no specific cure for PRS at present. After 7 years of progression from the onset, this patient entered a stable phase. Reconstructive treatment was then indicated, since there were no further signs of atrophy. Since adipose tissue autografts require complicated surgical skills, which take a lot of time and cost, the authors report a case of PRS augmented by HA filler in a 35-year-old male patient to suggest that HA filler could be a safe and simple alternative to surgical treatment. The authors conclude that HA fillers are a safe tool for treating facial deformities in patients with PRS.
Key Points
1. Hyaluronic acid (HA) fillers can be used for non-cosmetic purposes. HA fillers can be used to reconstruct facial features that have been altered due to congenital abnormalities. For instance, in patients with facial deformities resulting from conditions like Parry–Romberg syndrome, HA fillers can be injected to restore facial symmetry.
2. HA fillers are designed to imitate the densities of the tissues where they will be injected, providing a natural-looking result, with the possibility of being removed with hyaluronidase, an enzyme that can dissolve HA if any complications arise, or if a patient is not satisfied with the results.
3. Extensive anatomical knowledge, including vascular, tissue, and nerve anatomy, is crucial for safe and effective injection of HA fillers. It reduces the risk of complications and help achieving satisfactory results.
INTRODUCTION
First described by Parry in 1825 and Romberg in 1846, this variety of craniofacial findings was labelled as progressive hemifacial atrophy by Eulenberg in 1871. Other names used to describe this disorder include Parry–Romberg syndrome (PRS), idiopathic hemifacial atrophy, progressive facial hemiatrophy, and Romberg’s syndrome. Evidence of the disease dates back to Ancient Egypt, with mummies presenting craniofacial dysmorphism consistent with progressive hemifacial atrophy.1-3
PRS is a rare disease that leads to severe disfigurement after years of progressive hemifacial atrophy, with an estimated prevalence of 1 out of 700,000 people. It affects three times more females than males.4,5 Most patients have initial manifestations in the first two decades of life; however, late presentations are also described.3,6-8
A Mayo Clinic study of 54 patients showed an average age of onset of the disease of 13.6 years with a median of 10.5 years and a range of 0.3–75.0 years. These findings have been corroborated by other large reports.9-12 After the initial presentation, the disorder usually progresses over a 2–10-year self-limited period before spontaneous stabilisation.13-15 PRS can be categorised as mild, moderate, or severe.16 Mild disease is described as skin and subcutaneous tissue atrophy. Skin and soft tissue involvement manifest on the face, caudal to the forehead. Atrophy begins superficially, although the epidermis is minimally affected. Atrophy may progress to involve fascia, muscle, cartilage, and bone. Mandibular atrophy may be seen in severe cases.3
The pathogenesis of PRS remains unknown, but trauma, autoimmunity, infection, and autonomic dysregulation have all been suggested.17-19 Neurologically-based theories suggest that PRS may result from disordered developmental migration of neural crest cells, neurotrophic viral infection, trigeminal peripheral neuritis,16,20 or peripheral sympathetic nervous system dysfunction after traumatic disruption of the cervical plexus or sympathetic thoracic trunk.21 Systemic associations of PRS are variable, but neurological manifestations of seizures and headaches are common.3
The diagnosis is made on a clinical basis, but can be supported by imaging and skin biopsy. In some cases, the first symptom represents morphoea en coup de sabre, a unilateral frontal or frontoparietal impression of skin and underlying tissues, with scarring of the dermis.22 Children with morphoea en coup de sabre and PRS present with neuroimaging findings.23,24 PRS has a significant impact on social and life functioning.
The major aesthetic goal of treatment for patients with PRS is the restoration of facial contour and symmetry. Reconstruction often begins with autologous or synthetic fillers, and continues with surgery when indicated for the extent of tissue involvement. Injectable calcium hydroxyapatite, poly-L-lactic acid, silicone, and polyethylene have been used to correct mild or moderate soft tissue atrophy successfully. This paper aims to present the authors’ experience using reticulated hyaluronic acid (HA) fillers for facial augmentation.
CASE REPORT
The authors present a 35-year-old male with right hemifacial atrophy involving the right temporomalar area pre-auricular area extending to the right side of the jaw, sparing the mouth. The other side of the face appeared intact. Figure 1 shows pre-procedure photographs.
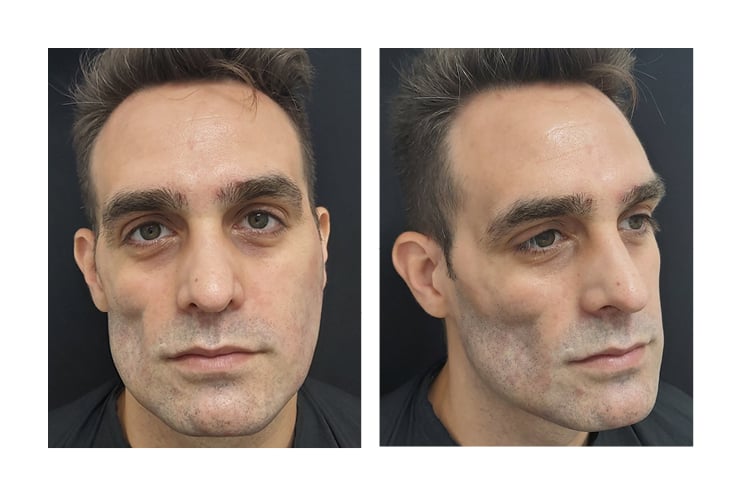
Figure 1: Pre-procedure photographs.
The onset has been estimated at the age of 20, demonstrating gradual progression over the years, leading to considerable wasting of the right side of the face. The patient reported the lesion to have been stable in appearance for the last 8 years.
The psychological distress caused by this condition affected the patient’s quality of life, limiting their inner circle to their closest contacts, and working only virtually. The patient came to the authors’ clinic to seek reconstructive treatment.
A diagnosis of PRS was made based on the clinical picture. Since the lesion had been stable at the consultation time, the authors planned treatment with HA fillers. HA is a good option for many pathologies presenting with unsightly anatomical alterations.25 The treatment was carried out in four sessions. Initially, the authors focused on the recovery of the structure, and then on the refinement and details. Treatment consisted of 12 syringes of cross-linked HA of different concentrations (17.5 and 20 mg/mL).
27G and 30 G needles and 22 G cannulas were used because they are safer.26 The skin was cleaned with alcoholic chlorhexidine because it is an antiseptic with excellent coverage and a low rate of adverse effects.27 Figure 2 shows post-procedure photographs.
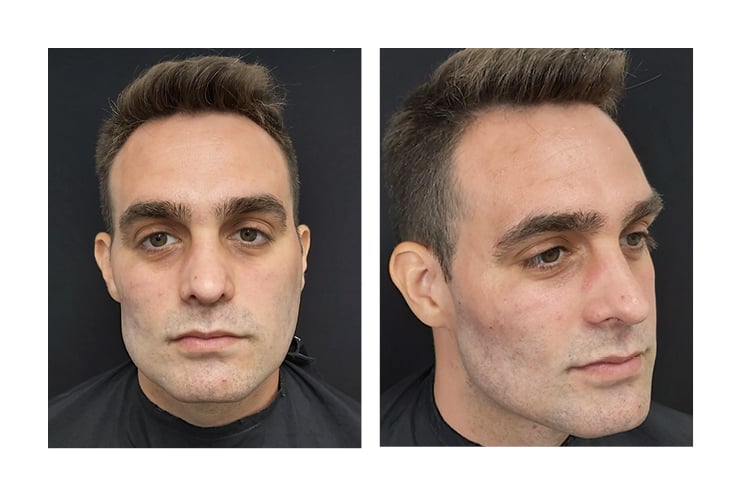
Figure 2: Immediate post-procedure photographs.
DISCUSSION
PRS is mainly a clinical diagnosis, since no established diagnostic criteria exist. A complete history of the disease, a detailed physical examination, consideration of possible differential diagnoses, and complementary studies are required.28 PRS should be suspected in cases of unilateral generalised soft tissue atrophy with thin skin that is not preceded by inflammation or induration.28
PRS affects the dermatomes of one or more branches of the trigeminal nerve, leading to a progressive shrinking and deformation of one side of the face. PRS results in unilateral facial atrophy and deviation of the mouth and nose to the affected side, compromising the aesthetics and the functionality of the face.21
Guerrerosantos et al.29 classified PRS into four types: Types 1 and 2 involve a decrease in facial soft tissue, while Types 3 and 4 present soft tissue thinning, as well as bone and cartilage atrophy. Type 1 is the mildest form and Type 4 is the most severe.30
Histopathological examination of skin affected with PRS usually reveals atrophy of the epidermis, dermis, subcutaneous tissue, skin appendages, vessels, and/or hair follicles,19 and skin fibrosis with collagen fiber thickening and skin oedema.31,32 Inflammation in the form of lymphocytic infiltrates may be present. Degenerative alterations of the vascular endothelium have been identified in electron microscopy.19 Although the pathophysiology of this disease is not well known, trauma, infections, cranial vascular malformations, immune-mediated processes, neurological alterations in fat metabolism, and sympathetic dysfunction are considered possible origins of PRS.21 PRS has not only been associated with autoimmune diseases, congenital diseases, and pregnancy, but has also been related to neurological, ophthalmological, cardiac, rheumatological, infectious, endocrine, maxillofacial, and orthodontic manifestations.28
There is numerous treatment available for PRS, from medical treatments to surgical techniques. However, medical treatments are effective only in the progressive phase, and do not address facial deformities. Topical treatments include steroids and calcipotriol plus psoralen (with ultraviolet A). Systemic drugs are oral steroids, methotrexate, D-penicillamine, tetracyclines, and antimalarials (hydroxychloroquine or chloroquine).24 However, the authors’ literature review highlighted that most of the drugs used correspond to a series of cases and small non-randomised trials.33-36
Reconstruction often begins with autologous or synthetic fillers. The use of permanent fillers, semi-permanent fillers, and resorbable fillers has been described. For surgical reconstruction, autologous fat transplantation or surgical flap may be indicated.24,37-40
Autologous fat transplantation has several benefits compared with flap-based procedures, including a reduction in donor site morbidity, operative time, and lower incidence of complications. However, it can result in incision scars in the donor areas, overcorrection, and irregularities.37 In addition, in patients with PRS, ‘take fat’ (reabsorption of injected fat) is described in a greater proportion than in the general population, which makes a greater number of surgical interventions necessary.41 Long-term results of fat grafting are often disappointing because of the unpredictable rate of reabsorption. Chang et al.42 associated lipotransfer with stromal vascular fraction, proving that it is effective and safe for the treatment of PRS, as it is able to improve the survival of grafts in the face without major complications.
In cases of severe bony abnormality, surgical flaps are indicated. Surgical flaps include free vascularised flaps43,44 and local pedunculated flaps.45 Other therapeutic possibilities are bone and cartilage grafts, which are used in advanced stages of the syndrome where these structures are affected, and also dermo-fat grafts obtained from the inguinal area.29
There are also treatments described where porous polyethylene implants are associated in the malar region as a complementary treatment to lipotransfer,46 and others where the placement of Integra® Dermal Regeneration Template (Integra LifeSciences, Princeton, New Jersey, USA), a bilaminar dermal regeneration template with an upper layer of silicone sheet that imitates the epidermis, was associated with platelet-rich plasma.47 Surgery generally requires a longer recovery time and risk of complications, such as flap failure.48
Several cases of PRS treated by semi-permanent and permanent fillers have been reported, including calcium hydroxyapatite (CaHA).49 The use of permanent fillers like Poly(methyl methacrylate) has also been described,50 and polyacrylamide hydrogel (PAAG) filler.51 In the case of CaHA and polyacrylamide, the goal is to provide immediate volume and support while stimulating dermal fibroblasts.52 CaHA has a good volumising effect due to collagen stimulation. PAAG filler is composed of 97.5% water and 2.5% cross-linked PAAG. Like CaHA, PAAG induces the formation of connective tissue.51 It has a longer-lasting effect than HA. However, neither of these products can be reversed in the event of inadvertent nerve compression or intravascular injection. Rees et al.53 reported excellent results with liquid silicone. However, over the years, several complications were described using this material, such as chronic cellulitis, nodules, foreign body reaction, and product migration, which were not related to the technique and, in most cases, were impossible to reverse.54
HA has also been used to correct atrophy in PRS patients.48,55-58 HA is a non-permanent, resorbable filler, and has replaced other standard filler materials.59 HA has an identical chemical composition in all species and tissues.60 Therefore, it has a less antigenic effect and less frequency of hypersensitivity reactions than products of animal origin.61
HA treatment carries advantages over a fat transfer since it can be performed in stages, avoiding overcorrections seen with lipotransfer.37 In addition, in the event of an inadvertent intra-arterial injection, hyaluronidase can reverse this infrequent complication.62-64
The authors proposed a combined technique to treat the temporal fossa using a 27 Gx1/2 needle for deep injections and a 22 G cannula for a superficial plane. The authors prioritised injections in the supra-periosteal plane in the so-called ‘swift point’,65-68 with volumising products of 20 mg/mL of HA. Veins and arteries run in a deep plane in the temporal fossa, so the authors decided to use a 22 G blunt cannula in the subcutaneous plane with a fan technique. The cannula always runs superficially, remembering that in the first fascia the anterior or frontal branch of the superficial temporal artery is found.69 The authors opted for a 17.5 mg/mL HA filler for superficial injections since it is easier to accommodate in such a shallow plane, where any imperfections are visible.
At the level of the lateral malar region, close to the temporomandibular joint, a 20 mg/mL HA filler with a 27 Gx1/2 needle was used with a bolus technique in a subperiosteal plane. The results were then optimised with a 17.5 mg/mL product of HA, using a 22 G cannula and a fan technique under the superficial musculoaponeurotic system.70
When injecting the preauricular or parotid region, the authors opted for 17.5 mg/mL HA products and a 22 G cannula, with a fan technique and in the hypodermic plane. This region presents the difficulty of massaging and accommodating the filling if necessary since in depth hard structures are lacking, being positioned on the parotid gland.71-73
For the region corresponding to the submalar triangle, the authors chose 20 mg/mL HA products and a 22 G blunt cannula. The technique used was the fan in the hypodermic plane, with subsequent massage of the treated area, inserting a finger inside the patient’s mouth.74,75
It was also necessary to approach the right mental region. For this, the authors used 20 mg/mL products, initially approaching the supra-periosteal plane with a 27 G needle76 and then the hypodermic plane with a 22 G blunt cannula. It must be remembered that the submental artery runs along the mandibular border in a deep plane, and in the case of intravascular injection, it can generate necrosis of the middle of the tongue.77
CONCLUSIONS
The authors report a case of PRS augmented by HA filler in a 35-year-old male patient to suggest that HA filler could be a safe, simple, alternative to surgical treatment.
The authors opted for cross-linked HA for its longer duration, various densities, and reversibility potential in an inadvertent intravascular injection. HA can be successfully used as a non-surgical alternative to correct facial deformities. It is an effective technique leading to cosmetically acceptable results. It is a reversible and relatively simple technique.
For all of the above, the authors concluded that HA filler would be the safest and easiest way to the correct mild to moderate atrophy in patients with PRS. Although they concluded that HA fillers are a safe tool for treating facial deformities in patients with PRS, they emphasise the importance of in-depth knowledge of facial anatomy for safer aesthetic injections, and to manage possible complications. It must not be forgotten that intravascular of the temporal fossa can lead to skin necrosis or blindness.






