Abstract
Chronic urticaria (CU) is characterised by intense recurrent itch, wheals, and/or angioedema, persisting for >6 weeks. CU can be subdivided into chronic spontaneous urticaria and chronic inducible urticaria; the latter usually appears with physical stimuli, such as heat, cold, pressure, and sunlight. The recommended treatment for CU is non-sedating oral antihistamines, administered up to four times a day. The monoclonal antibody omalizumab (anti-IgE) is recommended as an add-on therapy for patients with antihistamine-refractory CU. The fluctuating nature of urticaria symptoms and varying response to omalizumab often makes it difficult to predict the response to omalizumab; this often leads to individualised dosage regimens for CU patients. However, being able to predict the response to omalizumab treatment would lead to an improvement in dosage regimens and treatment plans in the clinical setting. Several studies have investigated potential CU biomarkers; however, no reliable biomarkers have been discovered that can be used to assess the treatment response to omalizumab in the clinic. Some potential biomarkers, such as plasma D-dimer, serum total IgE levels, the basophil histamine release assay, the autologous serum skin test, and the basophil activation test, have been suggested for predicting disease activity and response to omalizumab but are not implemented routinely in clinical practice. This paper presents an overview of the various biomarkers associated with response to omalizumab in CU.
INTRODUCTION
Urticaria is an itchy skin disease characterised by wheals and/or angioedema. Urticaria can be acute or chronic depending on the persistence of symptoms. In chronic urticaria (CU), symptoms are present for >6 weeks. CU can be subdivided into chronic spontaneous urticaria (CSU) or chronic inducible urticaria; the latter usually appears in response to physical stimuli, such as heat, cold, or sun exposure, or following the application of pressure.1 The most common type of non-acute urticaria is CSU, which has an estimated prevalence of 0.5–1.0% in the general population.2 Females are affected twice as often as males, with the highest incidence of CU seen in patients between the ages of 20 and 40 years; the average duration of CU is 3–5 years.2 The recommended treatment for CU is oral non-sedating antihistamines, taken up to four times a day, while omalizumab is used in cases of antihistamine-refractory CU.1
Omalizumab is a humanised monoclonal antibody that inhibits the binding of IgE to the high-affinity receptor FcεRI on the surface of basophils and mast cells;3 thus, omalizumab reduces the levels of free IgE and downregulates IgE receptors on these cells. This modulation of FcεRI receptors plays an active role in the clinical management of CU with omalizumab. However, the fluctuating nature of urticaria symptoms and the varying effect of omalizumab often makes it difficult to predict the course of treatment. Some biomarkers have been associated with response to omalizumab in the clinical setting and this review presents an overview of these available biomarkers.
TREATMENT OF CHRONIC URTICARIA WITH OMALIZUMAB
Several clinical studies have established that omalizumab significantly reduces urticaria symptoms in CU patients.4-7 Furthermore, omalizumab reduces the need for additional medication, improves patient quality of life, and reduces the number of days of urticaria symptoms.8 Real-world studies of antihistamine-refractory patients with CU treated with omalizumab have reported similar observations as randomised clinical trials in terms of the efficacy and safety of omalizumab.9
The recommended dose of omalizumab is 300 mg every 4 weeks and around 65% of patients experience a complete or almost complete response.4-7 Most patients experience flare-ups if administration of omalizumab is delayed by >30 days.10 Although omalizumab has a significant effect on urticaria symptoms, patient response patterns vary; fast responders experience a response within 2–4 weeks after initiating treatment, while some patients experience a slower response, which is seen 12–16 weeks after initiation of treatment. Therefore, it is recommended that omalizumab treatment is continued for at least 6 months before considering other options.11 However, no formal recommendation for the tapering or optimisation of omalizumab treatment exists when symptoms are well-controlled or reoccur. Nevertheless, in the case of symptom reduction, prolonging treatment intervals has been suggested in a treatment algorithm.12 Relapse of urticaria symptoms is often seen 2–8 weeks after the last injection of omalizumab; in such cases, retreatment with omalizumab has obtained good results.13 The most commonly reported adverse effects with omalizumab are headache, injection site itch and redness, and nausea.4-7 No severe adverse effects or complications to omalizumab have been reported.
Assessment of response to omalizumab treatment is usually based on overall physician assessment or validated patient-reported outcomes (PRO),14 such as Urticaria Activity Score in the past week (UAS7), which prospectively documents the intensity of itch and number of wheals daily on a scale from 0 (none) to 3 (severe) for 7 days. A UAS7 score ≥28 (score range: 0–42) indicates severe disease activity, while a UAS7 score ≤6 indicates well-controlled disease. The minimal clinical difference in UAS7 is equivalent to 10 points.15,16 Urticaria Control Test (UCT) is a retrospective questionnaire (score range: 0–16) that assesses disease control in the last 4 weeks. A score ≤11 indicates poor disease control, while a score ≥12 indicates well-controlled disease.17 The minimal clinical change in UCT is 3 points.18 Other PRO, such as Dermatology Life Quality Index (DLQI) and Chronic Urticaria Quality of Life Questionnaire (CU-QoL), are used to evaluate the impact of CU on the quality of life of the patient.19,20 These validated scores are of great value when monitoring urticaria patients, but a major disadvantage is that the scoring systems are subjective.
BIOMARKERS AND RESPONSE TO OMALIZUMAB IN CHRONIC URTICARIA
The fluctuating nature of CU symptoms and the varying response to omalizumab often leads to individualised dosage regimens for CU patients, making it difficult to predict response to treatment. Predicting omalizumab treatment response and changes in disease activity or severity would contribute to the development of a consensus treatment algorithm for clinical use and work as an objective follow-up tool for patients with fluctuating disease activity.14 Potential biomarkers for CU have been investigated in several studies (Table 1);21-28 however, there are currently no reliable biomarkers that can be used to assess the treatment response to omalizumab in the clinic. Some studies have suggested potential biomarkers, such as D-dimer, IgE levels, and the basophil histamine release (HR) assay, for predicting disease activity and response to omalizumab; however, none of these are currently implemented routinely in clinical practice.14,29
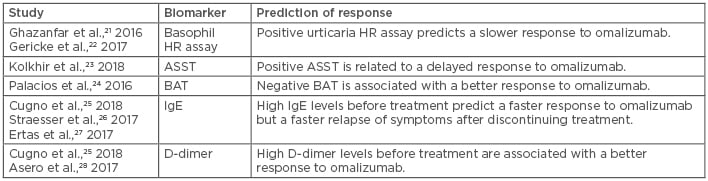
Table 1: Biomarkers associated with response to omalizumab in chronic urticaria.
ASST: autologous serum skin test; BAT: basophil activation test; HR: histamine release.
OMALIZUMAB AND AUTOIMMUNITY
One of the most frequent causes of CU is thought to be autoimmunity. Autoantibodies against the high-affinity IgE receptor or to autoantigens have been described as possible causes for CU;1 however, the pathological mechanism is not completely understood. Several laboratory tests can be used to measure autoimmunity in CU such as the basophil HR assay, autologous serum skin test (ASST), and basophil activation test (BAT). A positive basophil HR assay is often linked to autoimmune CU, treatment response, and disease activity in CU patients,30 and is defined as when HR from stimulated and unstimulated cells is >16.5% in both children and adults.31
A retrospective Danish study21 included 154 antihistamine-refractory CU patients from 2010–2014 and showed that a larger fraction of patients with a negative basophil HR assay had a complete or almost complete response to omalizumab compared to patients with a positive HR assay (77.3% versus 27.3%; p<0.01). However, in a 6-month prospective study of 117 CSU patients treated with omalizumab, the HR assay result was not predictive for omalizumab response measured with various PRO (UAS7, UCT, and DLQI).32 In addition, other patient-specific factors such as age, sex, duration of symptoms, presence of angioedema, ethnicity, and previous use of antihistamines and immunosuppressant drugs were not significantly associated with response to omalizumab.32
In a German study of 64 CSU patients refractory to oral antihistamines, the authors investigated the relationship between the urticaria HR assay and response to omalizumab.22 All patients were treated with 300 mg every 4 weeks and the follow-up time was 12 weeks. A total of 56 patients responded to omalizumab and 8 patients were unresponsive at Week 12. A response to omalizumab within 8 days was classified as fast (n=39), while a response after 8 days was classified as slow (n=17). Excluding one patient among the fast responders who had a positive urticaria HR assay, it was seen that patients with a positive urticaria HR assay only responded to omalizumab after the second injection and thus a slower response to treatment was seen; the median response time in patients with a positive urticaria HR assay was 29 days compared to 2 days in patients with a negative HR assay.22 These observations indicated that having a positive urticaria HR assay may be predictive of a slow response to omalizumab.22
The ASST is also associated with autoimmune CU and response to omalizumab. In the aforementioned German study,22 an ASST was performed in 51 CSU patients. It was seen that CSU patients with a positive ASST responded slower to omalizumab treatment compared to patients with a negative ASST. A total of 33 patients were fast responders and 13 responded slowly to treatment. Of these 13 patients, 10 had a positive ASST. Additionally, a significant association was seen between a positive ASST and a positive basophil HR assay. ASST positivity has also been linked to higher levels of C-reactive protein (CRP) in urticaria patients. No studies specifically investigating CRP levels and response to omalizumab have been performed; however, CRP levels are often significantly higher among antihistamine-refractory patients and have therefore been linked to non-responsiveness to antihistamines.23 Contrary to this, a prospective study from Korea that included 75 CSU patients reported that ASST positivity was a significant predictor for well-controlled CU.33
Basophil activation, quantified by flow cytometry, has also been suggested as a potential biomarker for severity of CU and the success of omalizumab treatment. Most studies have used CD63 or CD203c as markers for effective basophil activation. In a recent study from Spain, 139 patients with CSU were included to assess the diagnostic usefulness of BAT in combination with ASST in CSU disease activity.34 It was observed that a positive BAT was significantly associated with a positive ASST; however, a positive ASST was not associated with positive BAT in the same way.34 In another study of 41 CU patients, it was seen that a lack of upregulated CD203c correlated with clinical response to omalizumab. Thus, a negative BAT might be predictive of a positive response to omalizumab.24
In summary, positive autoimmunity tests such as the basophil HR assay, ASST, or BAT might be predictive of a poorer response to omalizumab in CU patients.
OMALIZUMAB AND IGE
It is becoming increasingly clear that IgE-mediated autoallergy and IgG-mediated autoimmunity contribute to the pathogenesis of CU; however, there are still many aspects of the disease that need to be explained.35 Recent studies have shown that patients with IgG autoantibody-mediated CSU experienced a slow response to omalizumab compared to patients with IgE autoantibody-mediated CSU.35 Omalizumab is an anti-IgE that reduces the free level of IgE and downregulates IgE receptors on basophils and mast cells. Therefore, it is acceptable to consider IgE as a potential predictor for response to omalizumab. Serum total IgE is, on average, elevated in patients with CU.30
In a recent German study of 113 (74 females) antihistamine-refractory CSU patients,36 IgE levels were investigated before and after treatment with omalizumab. All patients were treated with 300 mg omalizumab every 4 weeks and clinical response was evaluated with UAS7. At Week 12, 43 patients showed complete response, 55 showed partial response, and 15 patients showed no response to omalizumab. High disease activity and presence of angioedema were more common in the non-responders. Furthermore, it was seen that non-responders had lower IgE levels at baseline and similar observations were made in other studies.25,26 A two-fold increase in IgE levels from baseline to 4-week follow-up was also shown in complete and partial responders; hence, higher levels of IgE after treatment with omalizumab were associated with greater reduction of disease activity at follow-up. Additionally, it was seen that patients with higher levels of IgE at baseline also experienced faster relapse of urticaria symptoms after discontinuing omalizumab treatment.27 Higher levels of IgE in patients prior to treatment with omalizumab can be used as a predictor of almost complete or complete responders.
OMALIZUMAB AND D-DIMER
In some CSU patients, activation of the coagulation cascade, specifically the tissue factor pathway, is observed, and studies have shown that D-dimer is related to disease activity in CU patients due to the activation of this cascade.25 D-dimer is a fibrin degradation product and its presence reflects the expression of tissue factor by eosinophils, the activation of the coagulation cascade, and thrombin generation. Thrombin generation increases the permeability and induces degranulation of mast cells, while eosinophil activation increases plasma levels of D-dimer.37 Elevated D-dimer levels are often associated with refractory disease and poor response to antihistamine treatment in CU.38
It is also reported that D-dimer levels correlate with UAS739 and some studies have indicated that D-dimer is associated with response to omalizumab therapy. One study from Italy investigated D-dimer levels before and after treatment with omalizumab in 25 CSU patients with severe disease activity.25 Cugno et al.28 reported that baseline D-dimer levels were significantly lower in non-responders compared to partial and complete responders. In another recent study from Italy,28 32 antihistamine-refractory CU patients were treated with 300 mg omalizumab every 4 weeks for 3 months. A total of 75% of the patients reported a complete response to omalizumab. D-dimer levels were elevated in almost 60% of the patients and most of the patients with elevated D-dimer levels experienced complete response to omalizumab. Furthermore, an increase in D-dimer levels after administration of omalizumab was seen among non-responders.28 These studies indicate that elevated levels of D-dimer before treatment are associated with better response to omalizumab compared to patients with lower levels of D-dimers.
DISCUSSION
Although there is little literature investigating potential biomarkers associated with response to omalizumab in CU, the available studies suggest that several biomarkers used in clinical practice, such as the basophil HR assay, ASST, BAT, serum levels of IgE, and plasma D-dimer levels, are all associated with response to omalizumab in CU patients. For example, some studies have suggested that a positive urticaria HR assay is a marker of autoimmunity in CU and might be useful for predicting a less favourable treatment response to omalizumab.22,30 In contrast, a positive urticaria HR assay has also been associated with frequent spontaneous remission of CSU at 12 months and severe disease activity at onset.40 It has also been observed that a positive ASST, another marker of autoimmunity in CU, is predictive of a slow response to omalizumab.22 However, in one study ASST was described as a potential predictor for well-controlled CU.33 Furthermore, a positive BAT has been associated with poor response to omalizumab.24,34
It has been suggested that high baseline levels of serum total IgE are linked to a favourable response to omalizumab but also to faster relapse of symptoms after discontinuing treatment with omalizumab compared to patients with low IgE levels before treatment.25,26 Low levels of D-dimer before treatment were seen among non-responders to omalizumab, while elevated levels of D-dimer before treatment seem to be predictive of a positive response to omalizumab in CU patients.28
Recently, comprehensive proteomic profiling extending beyond single serological biomarkers has gained increasing popularity in possibly predicting disease activity and treatment response in CU. A recent study from Korea investigated differentially expressed proteins in the sera of CSU patients with positive (n=3) and negative (n=3) ASST and the correlation with disease control.41 In the ASST-positive group, the investigators identified seven upregulated proteins (apolipoprotein E-precursor, apolipoprotein J/clustrin, haptoglobulin, α-1-acid, glycoprotein, dynein heavy chain 8, and 8 albumin-like protein) and five downregulated proteins (two cleaved antichymotrypsins, plectin, polycomb protein SCMH1 isoform f, and α-1-ß-glycoprotein). Furthermore, the immunoassay of serum clusterin involved in cytoprotection against oxidants in ASST-positive and ASST-negative patients disclosed that clusterin levels were significantly higher in patients with ASST positivity compared to patients with negative ASST. It was seen that patients with higher levels of clusterin responded better to antihistamine treatment.41
Furthermore, autoallergic mechanisms in CU have been suggested because of the efficacy of omalizumab and increased levels of IgE in CU patients. In a German study,42 autoallergic targets of IgE were investigated in 1,062 CSU patients. Although >200 IgE autoantibodies were identified in CSU patients, it was noted that only IgE autoantibodies to IL-24 were found in all CSU patients. In these patients, IL-24 was associated with HR, disease activity, and reduced basophil count.42 Thus, the presence of IL-24 and elevated levels of clusterin might be useful in predicting response to omalizumab treatment in CU patients; however, more studies are needed to investigate this further before it can be translated into clinical practice.
CONCLUSION
In conclusion, the basophil HR assay, ASST, BAT, serum levels of IgE, and plasma D-dimer levels all show some usefulness in predicting treatment response to omalizumab in CU; however, they are not used regularly in daily clinical practice in all centres mainly because of the low-quality evidence in favour of their use. Future clinical studies are needed to identify new biomarkers in CU and provide evidence for their usefulness as tools in the management of CU in clinical practice.







