Abstract
With the recent advancements of biologic therapies that block IL-23, there is increasing need for analysis of which biologics are most efficacious in treatment of plaque psoriasis. Guselkumab and risankizumab have each individually been compared to adalimumab in head-to-head trials, but no prior clinical trials have directly compared them to each other. The authors performed a literature review of guselkumab and risankizumab to determine which treatment is more efficacious in the management of plaque psoriasis. Using PubMed, a literature review was conducted using the terms “adalimumab psoriasis”, “risankizumab psoriasis”, and “guselkumab psoriasis”. Fifteen studies resulted, and all were nonduplicate clinical trials written in English that were conducted within the past 5 years and included plaque psoriasis in the title. The data supports that risankizumab is more effective in improving Dermatology Life Quality Index (DLQI), static Physician’s Global Assessment (sPGA), and Psoriasis Area and Severity Index (PASI) 90 scores. However, risankizumab may be associated with more adverse events than guselkumab. Major limitations of this review include that only one prior head-to-head trial comparing risankizumab to adalimumab has been conducted and there are no Phase II studies comparing the two biologics. Furthermore, risankizumab is a recently approved treatment and data regarding long-term efficacy and side effects are limited. Risankizumab and guselkumab are both highly effective, very safe, and very convenient psoriasis treatments that can be considered first-line treatment options for patients with moderate-to-severe plaque psoriasis.
INTRODUCTION
Psoriasis is an immune-mediated disease that causes excessive keratinocyte proliferation resulting in erythematous, irritating lesions.1,2 Critical cytokines involved in the pathogenesis of psoriasis include TNF-α, IL-23, and IL-17.1 IL-23 plays a large role in supporting maintenance and survival of T helper 17 cells, which produce proinflammatory cytokines IL-22 and IL-17, contributing to the development of psoriasis.1-3
Plaque psoriasis is the most common variant of psoriasis and accounts for 75–80% of patients.4,5 Approximately 100 million individuals worldwide are affected by psoriasis and many patients experience reduced quality of life and negative psychological impacts attributable to the pain, scaling, and pruritus from the disease.1,2,6,7
Psoriasis therapies range from topical treatments in mild limited disease, to systemic treatments in severe disease.8 Topical therapy is indicated for psoriasis affecting <5% of total body surface area (TBSA) without involvement of the feet, hands, genitals, or face.9 Systemic therapy or ultraviolet-based therapy is indicated for psoriasis affecting ≥5% of TBSA.9 Approved nonsystemic therapies include phototherapy and topical agents such as corticosteroids and vitamin D3.10 Systemic therapies include biologics as well as nonbiologic agents such as cyclosporine, methotrexate, acitretin, tofacitinib, and apremilast.10,11
Biologics are the most recent advancement in the management of psoriasis and they exert their effect via inhibition of TNF-α, IL-23, or IL-17.12 Adalimumab, a TNF-α inhibitor approved in 2008 to treat plaque psoriasis, is one of the most commonly used biologics.1 Ixekizumab and secukinumab are two biologics that block IL-17. While these drugs are very effective, drugs that block IL-23 are among the most promising psoriasis treatments.1,3,7 Many genes associated with psoriasis correspond to the genes for the two subunits of IL-23 (p40 and p19) and the genes for the IL-23 receptor.1 Drugs that block IL-23 require few injections (as little as every 3 months), are very effective, and have proven very safe in both clinical trials and large registries.1,3,7,11
The first approved IL-23 blocker was ustekinumab, an antibody directed against the p40 subunit of IL-23 (the drug also blocks IL-12 as p40 is a subunit of both cytokines).3,11 Ustekinumab is dosed every 3 months, achieves a 75% improvement in Psoriasis Area and Severity Index (PASI) at 3 months in about 70% of patients, and has a strong safety track record.3,7,11 Nevertheless, IL-23 blockers based on p19 binding may replace ustekinumab in the treatment of psoriasis.3,7
Three p19-based IL-23 inhibitors are currently approved for psoriasis: guselkumab (first approved), tildrakizumab, and risankizumab (most recently approved). This study assessed the relative benefits and risks of these drugs using adalimumab as a common comparator. Tildrakizumab was excluded from this review as there is currently no direct comparison to adalimumab. Guselkumab and risankizumab both selectively bind to the p19 subunit of IL-23 and inhibit downstream intracellular signalling of IL-23 which helps prevent the role of inflammatory cytokines in psoriasis.1,6 While guselkumab and risankizumab have each been directly compared to adalimumab in the management of plaque psoriasis, no prior head-to-head comparison of guselkumab and risankizumab has been conducted to determine which biologic is more efficacious in the management of plaque psoriasis. This article evaluates the efficacy of guselkumab and risankizumab using a literature review of prior clinical trials analysing each biologic’s efficacy in comparison to adalimumab.
METHODS
A PubMed review of the literature was performed using the key terms “risankizumab psoriasis” or “guselkumab psoriasis” or “adalimumab psoriasis” (Figure 1). Results were further filtered and clinical trials were restricted to those written in English within the past 5 years (2015–2019). In total, this resulted in 78 articles. Articles that included plaque psoriasis in the title were further evaluated for a total of 15 articles. Additional results were obtained using the terms “guselkumab,” “adalimumab,” “risankizumab,” “ustekinumab,” and “tildrakizumab” to perform a more focussed search.
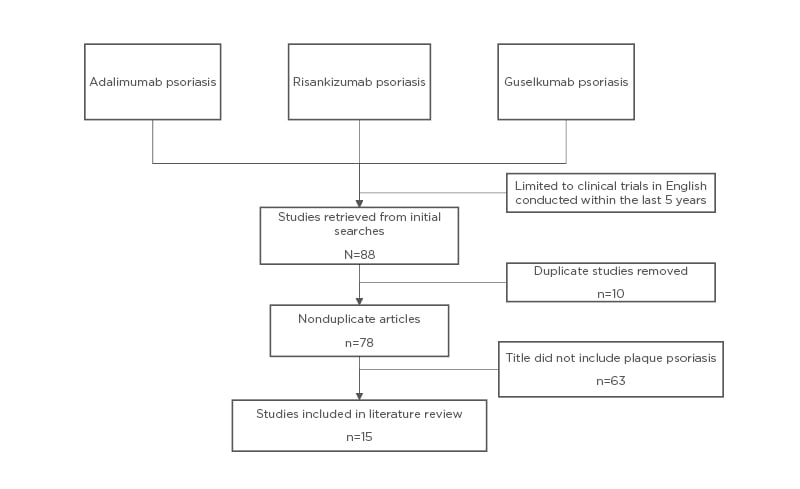
Figure 1: Literature review in PubMed.
RESULTS
Guselkumab Compared with Risankizumab
In a Phase III trial comparing guselkumab to adalimumab, 58.9% of guselkumab patients achieved a Dermatology Life Quality Index (DLQI) score of 0 or 1 at Week 24.6 DLQI assesses a patient’s perception of the effect of psoriasis on their daily life with scores ranging from 0 (no impact) to 30 (maximum impact).6 In a Phase III trial comparing risankizumab to adalimumab, more risankizumab patients (66%) achieved DLQI scores of 0 or 1 at Week 16.1 Additionally, 66% of the patients who were adalimumab intermediate responders (IR) and were rerandomised to risankizumab achieved DLQI scores of 0 or 1 at Week 44.1 It appears that risankizumab has a greater impact on quality of life scores and may lead to quicker improvement in scores than guselkumab (Table 1).1,2,6,13,14
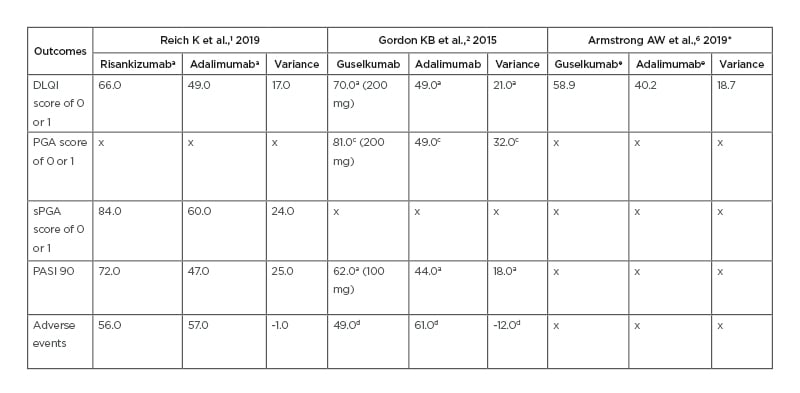
Table 1: Comparison of risankizumab, guselkumab, and adalimumab outcomes across three clinical trials.
All results are based on percent of people achieving each outcome. Variance refers to the difference in results between risankizumab or guselkumab and adalimumab.
aindicates outcome achieved at Week 16.
bindicates outcome achieved at Week 44.
cindicates outcome achieved at Week 40.
dindicates outcome achieved at Week 52.
eindicates outcome achieved at Week 24.
*indicates the referenced study results came from the original studies of Blauvelt A et al.,13 2017 and Reich K et al.,14 2017.
DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; PGA: Physician’s Global Assessment; sPGA: static Physician’s Global Assessment.
In a Phase II trial of guselkumab, 71% of patients in the 50 mg group, 77% in the 100 mg group, and 81% in the 200 mg group achieved a Physician’s Global Assessment (PGA) of 0 or 1 at Week 40.2 PGA score determines the degree of psoriasis involvement with scores ranging from 0 to 5.2 A score of 0 indicates cleared psoriasis, 1 minimal, 2 mild, 3 moderate, 4 marked, and 5 severe psoriasis.2 In a Phase III trial of risankizumab, 84% of patients achieved a static PGA (sPGA) score of 0 or 1 at Week 16.1 However, guselkumab had a greater difference from adalimumab on this variable (Table 2).1,2,6,13,14 Risankizumab, when dosed at 150 mg, is more effective in improving PGA scores than 50, 100, and 200 mg doses of guselkumab (Table 3).1,2
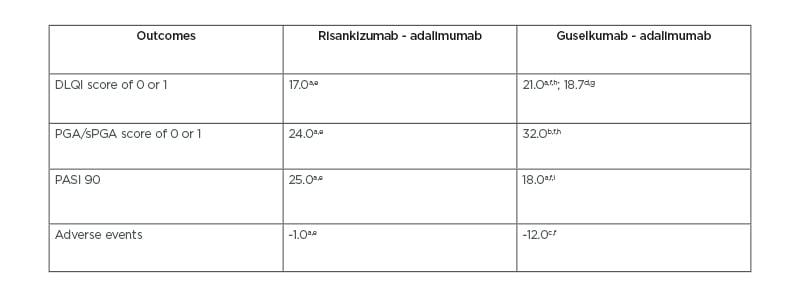
Table 2: Comparison of risankizumab and guselkumab variances from adalimumab across three clinical trials.
All results are percentages and reflect the variance between risankizumab or guselkumab and adalimumab. The variance was calculated from the percent of people achieving each outcome.
aoutcome achieved at Week 16.
boutcome achieved at Week 40.
coutcome achieved at Week 52.
doutcome achieved at Week 24.
eresults from Reich K et al.,1 2019.
fresults from Gordon KB et al.,² 2015.
gresults from Armstrong AW et al.,6 2019 which used data from the original studies of Blauvelt A et al.,13 2017 and Reich K et al.,14 2017.
ha dosage of 200 mg.
ia dosage of 200 mg.
DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; PGA: Physician’s Global Assessment; sPGA: static Physician’s Global Assessment.
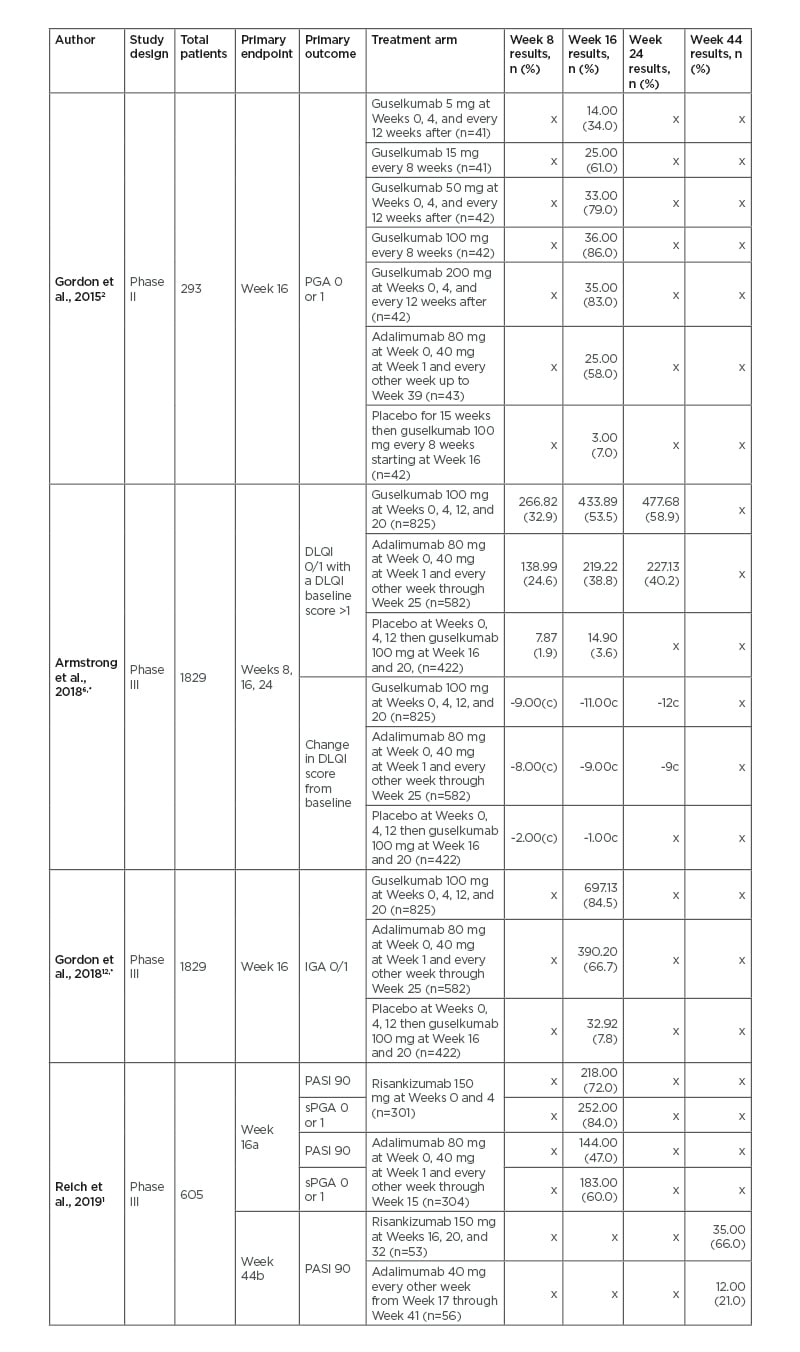
Table 3: Comparison of risankizumab and guselkumab to adalimumab in Phase II and III clinical trials.
apart A of study.
bpart B of study which analysed adalimumab intermediate responders.
cmedian change.
*the referenced study came from the original studies of Blauvelt A et al.,13 2017 and Reich K et al.,14 2017.
n the number of people achieving results in each category.
DLQI: Dermatology Life Quality Index; IGA: Investigator’s Global Assessment; PASI: Psoriasis Area and Severity Index; PGA: Physician’s Global Assessment; sPGA: static Physician’s Global Assessment.
In a Phase II trial of guselkumab, 34% of patients in the 5 mg treatment group, 34% in the 15 mg group, 45% in the 50 mg group, 62% in the 100 mg, and 57% in the 200 mg group achieved PASI 90 at Week 16.2 PASI is used to determine psoriasis severity. Scores range from 0 to 72, with higher scores indicating greater severity.2
In a Phase II study, 72% of risankizumab patients achieved PASI 90 at Week 16.1
Of the adalimumab IR rerandomised to risankizumab at Week 16, 66% achieved PASI 90 at Week 44.1 Risankizumab had a greater number of patients achieve PASI 90 at Week 16 than guselkumab (Table 3).
Differences exist when comparing guselkumab and risankizumab to IL-17 inhibitors in head-to-head trials. PASI 100 was achieved at Week 12 for 25% of patients randomised to guselkumab and 41% of patients randomised to ixekizumab with all major endpoints for ixekizumab having statistically significant greater improvement compared to guselkumab at Week 12.15 An additional study showed that 87% of risankizumab patients achieved PASI 90 at Week 52 compared to only 57% of secukinumab patients (p<0.001), with risankizumab having superiority compared to secukinumab for all secondary endpoints at Week 52 (p<0.001).16 Risankizumab may be a more effective treatment for achieving PASI 90 than IL-17 inhibitors with IL-17 inhibitors being more effective than guselkumab at achieving PASI 100.15,16
As with any medication, it is important to understand the risk of adverse events (AE). The most commonly reported AE in guselkumab patients were infections, but no serious infections were noted compared to a serious case of pneumonia in the adalimumab group.2 Serious AE (SAE) in the guselkumab group included one case of high-grade cervical cancer and one death from a myocardial infarction.2 In a Phase II trial, 49% of the patients receiving guselkumab reported AE at the end of 52 weeks.2 A Phase III study of risankizumab reported that 56% of the patients experienced AE at Week 16 and 76% of adalimumab IR patients, rerandomised at Week 16 to risankizumab, reported AE at Week 44.1 Furthermore, when comparing guselkumab and risankizumab to IL-17 inhibitors, 3% of both the guselkumab and ixekizumab groups at Week 12 experienced SAE with 5.5% of risankizumab patients and 3.7% of patients in the secukinumab group reporting SAE at Week 52.15,16 Guselkumab is less likely than risankizumab to cause AE when treating plaque psoriasis (Table 3) and risankizumab may be more likely to cause SAE than IL-17 inhibitors.15
A known difference between risankizumab and guselkumab is the convenience of dosing. Guselkumab requires starter doses at Weeks 0 and 4 with one injection every 8 weeks after starter injections are complete.11 In contrast, risankizumab requires starter doses at Weeks 0 and 4 with two-injection maintenance dosing every 12 weeks starting at Week 16. Although risankizumab requires two injections, the four yearly maintenance doses may be more appealing for patients compared to the six yearly maintenance doses of guselkumab.
Guselkumab Compared with Adalimumab
In a 52-week Phase II placebo-controlled, double-blind, randomised trial, guselkumab was compared to adalimumab in patients with moderate-to-severe plaque psoriasis.2 In total, 293 patients were randomised to receive either placebo, one of five guselkumab treatment regimens, or adalimumab (Table 3).1,2,6,12-14 To be included in the study, patients had to be ≥18 years and have experienced moderate-to-severe plaque psoriasis for at least 6 months. Moderate-to-severe was defined as a PGA score of ≥3, involvement of >10% TBSA, and a PASI score of ≥12. Patients previously treated with guselkumab or adalimumab were excluded from the study. The primary outcome was the achievement of a PGA score of 0 or 1 in patients at Week 16 (Table 1).2
Compared to adalimumab, the proportion of patients with a PGA score of 0 or 1 at Week 16 was higher for all guselkumab groups except for the 5 mg regimen group (Table 1). By Week 16, the 5, 15, 50, 100, and 200 mg guselkumab groups achieved PASI 90 in 34%, 34%, 45%, 62%, and 57% of the patients, respectively.2 By Week 16, 44% of the adalimumab group achieved PASI 90. By Week 40, the guselkumab groups had achieved a PGA score of 0 or 1 in 71%, 77%, and 81% of patients in the 50, 100, and 200 mg groups, respectively. In comparison, only 49% of the adalimumab group achieved a PGA score of 0 or 1 (p<0.05). During the first 16 weeks of the trial, infection rates for guselkumab and adalimumab were 20% and 12%, respectively.2 Fewer injection-site reactions occurred with guselkumab (1%) than adalimumab (6%). Fewer patients (49%) treated with guselkumab experienced AE from Week 16–52 of the study compared to adalimumab recipients (61%). No association was noted between the dose of guselkumab and increased rate of AE.2
In two 24-week Phase III placebo-controlled, double-blind, randomised trials, guselkumab was compared to adalimumab in patients with plaque-type psoriasis.13,14 Patients were randomised to receive either guselkumab, adalimumab, or placebo (Table 1). Inclusion criteria included age >18 years, diagnosis of plaque-type psoriasis for at least 6 months with 10% or more TBSA involvement, PASI score of 12 or higher, Investigator’s Global Assessment (IGA) score of at least 3, and eligibility for systemic or phototherapy treatments.13 Patients with a history of active tuberculosis, a progressive, uncontrolled, or severe medical condition, or a history of malignancy (except nonmelanoma skin cancer) within the previous 5 years were excluded from the study.13 Similarly, patients who received guselkumab, adalimumab, or another anti-TNF-α therapy in the past 3 months; IL-12/23, IL-17, or IL-23 inhibitors in the past 6 months; phototherapy in the past 1 month; or systemic immunosuppressant therapy in the past 1 month were excluded.13 Pooled data from the two 24-week trials were used to compose two studies which examined different endpoints and effects of the treatments.6,12
The primary endpoints of the first pooled study included the patient proportion with a DLQI score of 0 or 1 (no impact on quality of life) that had a baseline score greater than 1 and the proportion of patients with a DLQI score that changed from baseline to Weeks 8, 16, and 24 (Table 1).6
An additional endpoint was the proportion of patients with a DLQI individual domain score of 0 and the percent improvement in individual domains among patients with scores of 3 reflecting the most severe impact. At Week 24, a greater proportion of guselkumab patients (58.9%) achieved a DLQI score of 0 or 1 when compared to patients receiving adalimumab (40.2%; p<0.001).6 Guselkumab patients experienced greater improvement in individual DLQI domains at Week 24 compared to adalimumab patients (p<0.001). A greater proportion of patients (35.6%) treated with guselkumab at Week 24 achieved a Psoriasis Symptoms and Signs Diary (PSSD) symptoms score of 0 (free of symptoms) than those receiving adalimumab (22.0%; p<0.001).6 The proportion of patients who achieved a PSSD score of 0 was also higher for patients treated with guselkumab (28.4%) compared to adalimumab (15.6%).6
The primary endpoint of the second pooled study was an IGA score of 0 or 1 (cleared or minimal psoriasis) at Week 16 when compared to placebo (Table 1).12 Two of the major secondary endpoints included an IGA score of 0 (cleared) or IGA of 0 or 1 (cleared or minimal psoriasis) at Week 24 when compared to adalimumab.12 At Week 24, a statistically significant proportion of patients treated with guselkumab achieved IGA 0 or IGA 0 or 1 for all comparisons except for the African American or black subgroup which was small and included 12 guselkumab and 13 adalimumab patients, respectively.12 Additionally, clinical responses for baseline weight strata were higher for the guselkumab group compared to adalimumab at Week 24. Both guselkumab and adalimumab had lower response rates in patients who weighed more, but the response rates were more consistent for guselkumab. In individuals of any weight, the clinical response is more consistent with the 100 mg guselkumab dose than adalimumab which is less efficacious in patients of greater weight.12 Regardless of prior treatment, patients treated with guselkumab achieved statistically significant improvements in IGA 0 (52.1%) and IGA 0 or 1 scores (83.8%) compared to adalimumab patients (IGA 0 [30.2%] and IGA 0 or 1 [63.1%]) at Week 24.12
Risankizumab Compared with Adalimumab
No Phase II studies have been conducted comparing efficacy of risankizumab to adalimumab. One Phase III double-blind, randomised, active-comparator-controlled trial was conducted in patients with moderate-to-severe plaque psoriasis to evaluate the efficacy of risankizumab.1 This Phase III trial was the first head-to-head trial comparing risankizumab to adalimumab. The trial lasted 44 weeks and was composed of Part A (Weeks 0–16) and Part B (Weeks 16–44). During Part A, patients were randomised to receive either risankizumab or adalimumab (Table 1). In Part B, patients who were randomised to adalimumab in Part A and achieved PASI 90 remained on adalimumab treatment while those who achieved a PASI 50 or less were switched to risankizumab (Table 1). Patients receiving adalimumab in Part A who achieved greater than PASI 50 but less than PASI 90 (adalimumab IR) were rerandomised to continue adalimumab or were switched to risankizumab in Part B. Additionally, patients who were originally randomised to the risankizumab regimen in Part A remained on this regimen in Part B. Participant inclusion criteria included age over 18 years, 10% or more TBSA with moderate-to-severe plaque psoriasis that was stable for at least 6 months, PASI score of at least 12, sPGA of at least 3, and eligibility for phototherapy or systemic psoriasis treatment as well as adalimumab.1 Patients were excluded if they had non-plaque or drug-induced psoriasis, ongoing inflammatory active disease, prior exposure to adalimumab, use of restricted medications, chronic or active infections, or had a history of malignancy in the preceding 5 years (except nonmelanoma skin cancer or uterine cervix in situ carcinoma).1 Primary endpoints for Part A of the study were achievement of PASI 90 and achievement of sPGA score of 0 or 1 (clear or almost clear at Week 16 (Table 1).1 The primary endpoint for Part B of the study was achievement of PASI 90 among adalimumab IR at Week 44 (Table 1).
By the end of Week 16 (Part A), more patients randomised to risankizumab achieved PASI 90 (72%), sPGA score of 0 or 1 (84%), PASI 75 (91%), and PASI 100 (40%) compared to adalimumab (PASI 90, sPGA score of 0 or 1, PASI 75, PASI 100 of 47%, 60%, 72%, and 23%, respectively).1 Additionally, adalimumab IR at the end of Part A who were rerandomised to risankizumab had a larger proportion of PASI 90 (66%) and PASI 100 (40%) compared to patients rerandomised to continue adalimumab (PASI 90, PASI 100 of 21% and 7%, respectively) at Week 44. A greater proportion of patients receiving risankizumab achieved a sPGA score of 0 in both parts of the study than adalimumab patients (p<0.0001 for Part A and B). Starting at Week 8, the proportion of patients achieving PASI 90 was higher for patients receiving risankizumab compared to adalimumab (p=0.0012). Differences between risankizumab and adalimumab regarding proportion of patients achieving sPGA scores of 0 and PASI 100 became apparent at Week 8. In Week 44, patients rerandomised to risankizumab during Part B had a higher mean improvement (93%) compared to patients rerandomised to adalimumab (72%) during Part B.1
Quality of life improvement was greater for patients receiving risankizumab than those receiving adalimumab with a greater proportion of risankizumab patients having DLQI scores of 0 or 1 (66%) compared to adalimumab (49%) at Week 16.1 Additionally, adalimumab IR who were rerandomised to risankizumab at the end of Part A had a greater proportion of DLQI scores of 0 or 1 at Week 44 (66%) than patients rerandomised to continue adalimumab (29%). Adalimumab patients who achieved PASI 50 or less at the end of Part A and were switched to risankizumab experienced clinical benefit with 61% achieving PASI 90 and 63% achieving sPGA scores of 0 or 1 at Week 44. Patients who were treated with risankizumab continuously throughout the 44-week study and achieved sPGA score of 0 or 1 and PASI 90 at Week 16 were maintained until the end of the study. In Part A, 56% of risankizumab patients and 57% of adalimumab patients reported nonserious AE, most commonly headache and upper respiratory tract infection. In Part A, 3% of patients in both risankizumab and adalimumab treatment groups experienced serious AE. In Part B, 76% of patients rerandomised to risankizumab and 66% of patients rerandomised to continue adalimumab experienced AE, most commonly headache, back pain, arthralgia, and upper respiratory tract infection. SAE occurred in 6% of patients rerandomised to risankizumab and 4% of patients rerandomised to continue adalimumab. No serious infections occurred in patients rerandomised to adalimumab during Part B of the study, but one patient did report a serious AE of depression.
No cases of serious hypersensitivity or active tuberculosis were reported in the study and no events of opportunistic infections, death, malignancy, or major cardiovascular events occurred in Part B. In Part A, there was one major adverse cardiovascular event, one case of depression in the risankizumab group, and one case of oral candidiasis in the adalimumab group. Five patients receiving risankizumab reported hepatic events with one patient discontinuing the medication and three patients receiving adalimumab reported hepatic events. Three reported deaths occurred during the study, however, none of them were related to the study drugs. During Part B, a patient rerandomised to risankizumab developed latent tuberculosis and a patient who was continuously on risankizumab throughout the study experienced depression. Hepatic events were reported in one patient rerandomised to risankizumab and four patients rerandomised to adalimumab.1
Risankizumab and Guselkumab Head-to-Head Studies Compared to IL-17 Inhibitors
Two recent head-to-head trials of guselkumab to ixekizumab and risankizumab to secukinumab provide additional information on which IL-23 inhibitor is more effective when compared to IL-17 inhibitors.15,16 To further provide additional information on which IL-23 inhibitor is more effective when compared to IL-17 inhibitors these studies were analysed. The head-to-head comparison of guselkumab to ixekizumab concluded that the primary endpoint of PASI 100 was achieved by 25% of patients randomised to guselkumab and 41% of patients randomised to ixekizumab at Week 12.16 Furthermore, all primary and secondary major endpoints for ixekizumab had statistically significant greater improvement compared to guselkumab at Week 12.16 The frequency of SAE was 3% for both the guselkumab and ixekizumab groups at Week 12.16 New head-to-head Phase III data comparing risankizumab to secukinumab analysed the primary endpoint of PASI 90 at Week 52.16 Of the risankizumab patients, 87% achieved PASI 90 at Week 52 compared to 57% of secukinumab patients (p<0.001).16 The other primary endpoint was noninferiority of risankizumab to secukinumab at Week 16 using PASI 90. At Week 16, 74% of risankizumab patients achieved PASI 90 compared to 66% of secukinumab patients.16 Additionally, risankizumab exhibited superiority compared to secukinumab for all secondaryendpoints at Week 52 (p<0.001).16 SAE were reported for 5.5% of patients in the risankizumab group and 3.7% of patients in the secukinumab group.16
Tildrakizumab
Tildrakizumab was excluded from this review as there is currently no direct comparison of this IL-23 inhibitor to adalimumab. However, the reSURFACE 2 study concluded that a greater proportion of patients receiving tildrakizumab (61% in 100 mg group and 66% in 200 mg group) achieved PASI 75 compared to patients receiving etanercept (48%) at Week 12 (P<0.05).17 Improvements in PGA scores to 0 or 1 occurred more frequently in both tildrakizumab groups (66% in 100 mg group and 71% in 200 mg group) than the etanercept group (48%) at Week 28 (p<0.001).17 SAE were similar across the tildrakizumab groups (2% in 200 mg group, 3% in 100 mg group) and etanercept (5%) at the end of the study.17 Since the primary endpoints are different in the VOYAGE trials of guselkumab and the reSURFACE trials of tildrakizumab direct comparisons cannot be made.17,18 However, results of the VOYAGE and reSURFACE trials suggest that guselkumab may be more effective than tildrakizumab because a higher percentage of patients at Week 12 achieved a PGA score of 0 or 1, PASI 75, PASI 90, or PASI 100 for guselkumab groups when compared to tildrakizumab groups.18 Tildrakizumab is more effective than the TNF-α inhibitor etanercept at achieving PASI 75.18 Previous randomised controlled trials support that tildrakizumab is well tolerated for up to 64 weeks in large groups of patients with moderate-to-severe psoriasis.17,19,20 Future research is needed regarding tildrakizumab and how it compares in head-to-head trials to adalimumab and risankizumab.
CONCLUSION
With continued emergence of new psoriasis medications, there is an increasing need for analysis of which biologics are most efficacious in the treatment of plaque psoriasis. This clinical review compares guselkumab and risankizumab to provide some sense of which IL-23 inhibitor biologic is more effective in treating plaque psoriasis. Guselkumab and risankizumab have each individually been compared to adalimumab in head-to-head trials, but no prior clinical trials have directly compared them to each other.
A major limitation of this review is that only one prior head-to-head trial comparing risankizumab to adalimumab has been conducted and there are no Phase II studies comparing the two biologics. Furthermore, risankizumab is a recently approved treatment and data regarding long-term efficacy and side effects are limited. However, a Phase III trial comparing the efficacy of risankizumab to secukinumab (IL-17 inhibitor) is underway,21 and the resulting data will be helpful for comparing risankizumab to guselkumab using secukinumab as the common comparator.1 The indirect comparisons have the advantage of coming from studies in which there were also placebo-controls (head-to-head trials without placebo controls often find higher response rates than those with placebo controls). Additional limitations include lack of current research comparing tildrakizumab, the third IL-23 inhibitor, to adalimumab; therefore, this review was unable to directly compare risankizumab and guselkumab to tildrakizumab. While previous trials suggest that guselkumab may be more effective than tildrakizumab, further research is needed to compare tildrakizumab to adalimumab, guselkumab, and risankizumab to comprehensively evaluate the efficacy of IL-23 inhibitors.18
According to previous clinical trials, risankizumab is more effective in improving DLQI scores and PGA scores, and results in more patients achieving PASI 90.1,2,6 However, risankizumab may be associated with more AE than guselkumab.1,2 Additionally, although risankizumab requires two injections, the four yearly maintenance doses may be more appealing for patients compared to guselkumab’s six yearly maintenance doses.11 Psoriasis treatment targets of PASI 100/DLQI 0 or 1, and of PGA 0 have been promulgated.11,22 Both risankizumab and guselkumab can achieve these outcomes, though the difference in their ability to do so may not be clinically relevant. Risankizumab may be a more effective treatment for achieving PASI 90 than IL-17 inhibitors with IL-17 inhibitors being more effective than guselkumab at achieving PASI 100.15,16 Risankizumab and guselkumab are both highly effective, very safe, and convenient psoriasis treatments that can be considered first-line treatment options for patients with moderate-to-severe plaque psoriasis.