Abstract
Hidradenitis suppurativa (HS) is an inflammatory skin condition of the follicular pilosebaceous unit that primarily affects flexural areas where apocrine glands are found. This disorder can present as either an acute or chronic disease, with a single subcutaneous nodule or clusters of painful abscesses with purulent drainage in one or more of the following sites: axilla, groin, genital, perianal (more common in males), and under the breasts (more common in females). Over time patients form sinus tracts, fibrosis, and scarring. The onset usually occurs in the early 20s, after puberty. HS can be present for years without being diagnosed and is associated with a diminished quality of life, high morbidity, and substantial healthcare costs. Global HS prevalence is estimated at 1%.
This article reviews a retrospective cohort study of 13 patients assessed by an interprofessional wound care team and discusses relevant literature. Accuracy of referral diagnosis was the primary outcome. Secondary outcomes included demographics and quality of life. In total, 10 patients were female (77%) and the mean age was 33 years. Fewer than half (n=6, 46%) had an accurate diagnosis of HS prior to team assessment. Of these patients, the mean time before a correct diagnosis was 4.2 years. Untreated bacterial damage was diagnosed in the majority of patients (n=9, 69%). There was substantial improvement in pain levels and quality of life in approximately half of the cases. Over time, patients became more actively involved in their care. Our findings show HS diagnosis and management is optimised with an interprofessional team approach.
INTRODUCTION
Hidradenitis suppurativa (HS) is an inflammatory condition that affects the follicular pilosebaceous unit, especially where apocrine glands are found. The axilla, groin, buttocks, genital, inframammary (especially females), and perianal regions (especially males) are primarily affected.1 HS usually presents as subcutaneous nodules that become painful abscesses with purulent drainage, followed by the formation of sinus tracts, fibrosis, and scarring.2 Clinical manifestations are classified using the Hurley stages to direct treatment modalities.3 Severity is graded by a variety of different scoring systems, including the Sartorius score to monitor effectiveness of interventions in clinical trials (Figure 1).4,5
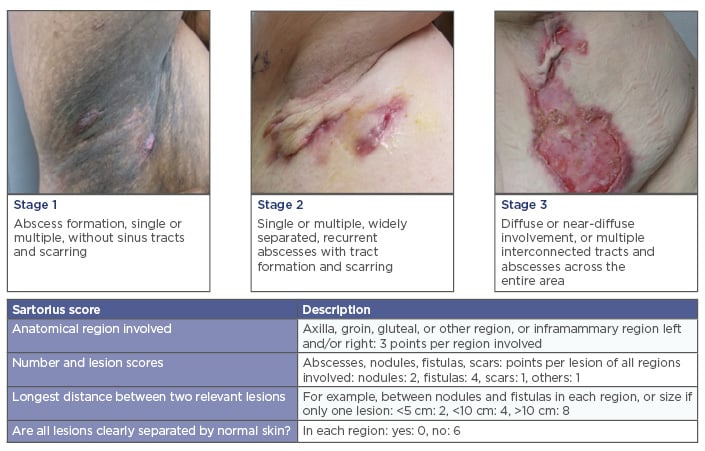
Figure 1: Hurley stages and Sartorius score.
PATHOGENESIS, PREVALENCE, AND RISK FACTORS
The pathogenesis of HS is thought to be multifactorial.6,7 Follicular occlusion and hyperkeratosis are considered the principal initiating causes.8 The disease has a diverse histological spectrum including: pore occlusion, sinus tracts, epithelial cysts, pyogenic granulomas, and scarring.9 HS is part of the follicular occlusion tetrad of diseases, along with acne conglobata, dissecting cellulitis of the scalp, and pilonidal sinus disease.7,9,10 The acute nodules and abscesses of HS usually start in the early 20s after puberty.2,11,12 The global prevalence of HS is estimated at 1%.2,13-16 A Canadian population-based survey of 10,200 people calculated the HS prevalence at 3.8%, with an annual incidence of approximately 30 new cases per 10,000 population over a 1-year period.17 Females are more commonly affected, with a female-to-male sex ratio of approximately 3:1.14,15,18
There are strong associations of HS with smoking, high BMI, family history, Type 2 diabetes mellitus, and thyroid disease.19 Various hormonal and immunological factors such as tumour necrosis factor (TNF) and the interleukin-23/T helper 17 pathway (IL-23/Th17) are involved, although the exact pathogenesis is unclear.19,20 Smoking is a particularly strong HS risk factor and increases the chance of symptomatic onset and greater severity. The percentage of active or previous smokers with HS has been recorded as ranging from 50–90%.21-23 A German matched case control study found that current smokers had a 9.4-times greater chance of HS compared to non-smokers or ex-smokers.22 A greater prevalence and severity of HS have also been reported in patients with high BMIs.5,21,24,25 Revuz et al.14 concluded that the relative HS risk increases by 12% for every unit increase of BMI>25. Another retrospective chart review study found 50.6% of patients with HS had metabolic syndrome compared with 30.2% in a matched control group without metabolic syndrome.25 Familial inheritance of HS is probably autosomal dominant with variable penetrance and expressivity. Approximately one-third of HS patients have a positive family history of HS,12,26 and this is often associated with an early onset.18,27,28 HS impacts patients’ quality of life in many ways including pain, exudative wounds, odour, shame, embarrassment, and restricted movement.29-33
DIAGNOSTIC AND TREATMENT CHALLENGES
Early diagnosis and treatment is challenging for clinicians due to the variety of phenotypes, complexity of HS, and lack of randomised controlled trials to guide treatment. A cross-sectional study of 80 Canadian patients with HS documented a 5-year average delay between presentation and diagnosis with some patients living undiagnosed for ≤25 years.31 This long delay between presentation to diagnosis and subsequent treatment increases associated healthcare utilisation costs, including emergency department visits, outpatient care visits, and diagnostic tests.29,34,35
Topical and systemic pharmacological treatments are recommended, including antimicrobials, intralesional corticosteroids, biologics, and, occasionally, anti-androgens.34,36,37 Anti-inflammatory antimicrobials, including tetracyclines and clindamycin, have been proven effective.38-40 Topical or systemic clindamycin or oral tetracycline (including doxycycline and minocycline) are effective for mild disease.5,41 Second-line therapies include: clindamycin with rifampin, oral retinoids (acitretin is more effective than 13-cis-retinoic acid), and intralesional steroids.34,39,41 For more severe cases biologics (TNF inhibitors including adalimumab and infliximab [review] and immunosuppressant agent azathioprine) are used.34 A Cochrane review of 12 randomised controlled trials (615 participants) reported there is moderate evidence of adalimumab and infliximab improving Dermatology Life Quality Index (DLQI) scores.42 There is also improved quality of life with the biologics.43 Deep and surrounding infection should be ruled out for any patient with HS receiving biological agents.
Surgical treatment should be assessed early depending on disease severity, anatomical location, and degree of scarring.41,44,45 Surgical methods include local destruction (cryotherapy, incision and drainage, punch debridement [with curettage], unroofing/deroofing including removal of amorphous debris in the base or else recurrences are common), and local or extensive excision.34,45
HS recurrence rates are influenced by many factors, including lifestyle, particularly smoking and obesity, disease severity, anatomical location, local wound care, and treatment. A systematic review and meta-analysis of 22 articles concluded that excisional surgery is the best treatment of severe but localised forms of HS with lower recurrence rates.46 Local excision with primary closure is associated with higher recurrence rates.45 Mandal and Watson47 reported a 69.9% recurrence rate after first surgical intervention with primary closure and low recurrence rates with wide excision and healing by secondary intention. Overall, the literature suggests a comprehensive management plan for HS lesions includes addressing general measures, appropriate use of topical and systemic antimicrobials combined with optimal local wound care, surgical debridement, and intralesional steroid injections (Table 1).34
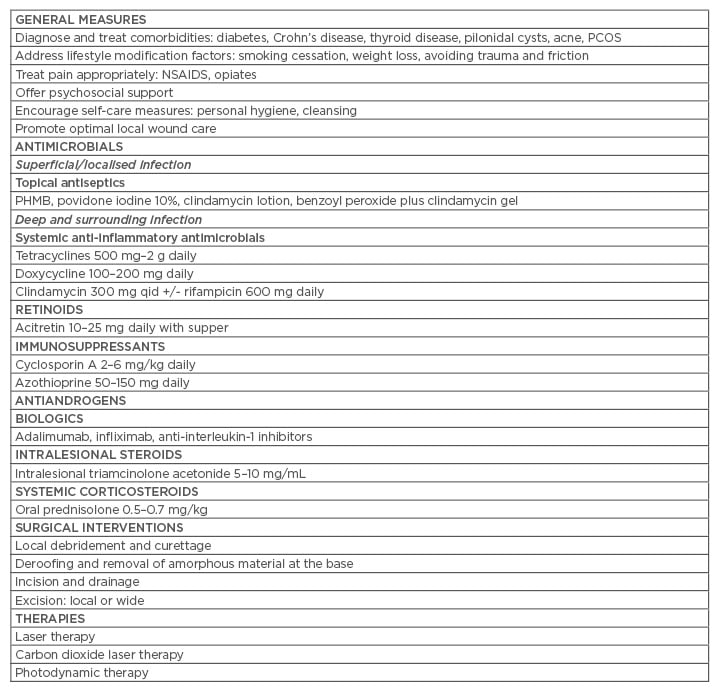
Table 1: Practice tips for managing hidradenitis suppurativa.
PCOS: polycystic ovary syndrome; PHMB: polyhexamethylene biguanide; NSAIDS: non-steroidal
anti-inflammatory drugs; QID: four-times daily.
INTERPROFESSIONAL APPROACH AND WOUND BED PREPARATION
An interprofessional approach, coupled with patient and clinician-directed educational interventions, is crucial for optimal management of HS and its complications.30,31,35 The Wound Bed Preparation paradigm is a comprehensive, systematic approach to patient management that addresses three main principles: treating the cause, patient-centred concerns, and local skin/wound care.48 Treating the cause and addressing patient-centred concerns are important to determine if there can be complete resolution of the lesions (healing ability). Wound and skin care can be determined as healable (often with definitive excisional surgery), non-healable (e.g. patient is a heavy smoker, obese, and/or not adherent to treatment), or maintenance (acute or subacute episodes that can be controlled). For local wound care, debridement, infection, and moisture balance must be addressed. The validated NERDS and STONEES criteria can be utilised to diagnose localised (superficial) or deep-surrounding infection.48,49 Patient-centred concerns commonly include pain and body image issues and should be addressed to ensure patient adherence to treatment. An interprofessional team that follows this paradigm can manage patients with HS in a holistic manner.
METHODS
Literature Review
The Cochrane Library, University of York Centre for Reviews and Dissemination database, and PubMed (Medline) databases were reviewed. The MeSH term ‘hidradenitis suppurativa’ was used in combination with the keywords ‘co-morbidities’, ‘chronic disease’, ‘wound care’, and ‘dermatology’. No limit was placed on the publication year and only English language articles were used based on reviewer judgement.
Study Design, Data Abstraction, and Analysis
A retrospective cohort study of 318 patients with complex and unresolved chronic wounds who were referred between February 2013 and September 2014 to an interprofessional team (Toronto Regional Wound Healing Clinic, Ontario, Canada). The team consisted of an internist/dermatologist physician with expertise in wound care (Dr Sibbald) and three nurses with extensive wound care proficiency. The team had strong communication with primary care physicians and family health teams with referral links to plastic and general surgery, infectious disease specialists, social workers, and certified diabetes educators. Initial comprehensive assessments were approximately 2 hours; follow-up assessments were approximately 30 minutes.
A case report form (CRF) was used to abstract data from paper charts. The CRF was collectively formulated and pilot tested by the study team. Data were abstracted by a physician (Dr Persaud) and information from the CRF was entered into an SPSS® (Version 23 IBM®) electronic database for analysis. Validation was performed by comparing a random sampling of 10% (n=30) of SPSS case entries against the original paper charts. No discrepancies were found.
Inclusion Criteria
This review was solely from the 13 patients who had a diagnosis of HS after their initial comprehensive interprofessional assessments (CIA). The diagnosis of HS was based on patient history and clinical presentation. The Trillium Health Partners Research Ethics Board (REB) ID 635 approved this study.
RESULTS
Diagnosis, Demographics, Duration, and Comorbidities
HS was diagnosed in 13 (4.1%) of the 318 referred patients with complex wounds. Less than half (n=9, 46%) had a correct diagnosis of HS at time of referral. For those who did not have a prior HS diagnosis (n=7, 54%), the mean time from onset of symptoms to diagnosis was 220 weeks (4.2 years). Patients were predominately female (n=10, 77%; sex ratio ˜3:1), the mean patient age was 33 years, and mean patient BMI was 31.8 (obese). Two-thirds of patients were current or past smokers (n=8, 67%), well above the average smoking rates for Canada. Mean age at HS onset was 22 years (range: 10–40 years). Comorbidities present were diabetes, hypertension, dyslipidaemia, and polycystic ovarian syndrome; all n=2 (15%). There was an almost even distribution between patients presenting as Hurley Stage 1 (n=6, 46%) and Hurley Stage 2 (n=7, 54%). Over two-thirds of patients (n=9, 69%) had a previous surgical intervention related to their clinical presentation.
Treatment Before and After Comprehensive Interprofessional Assessment
The average number of nursing visits was 3.7 per week (ranging from 0–8 per week). Enterostomal therapists were the most common referring professional (n=7, 54%). The cohort’s ability to heal was unknown for 6 patients (46%), but subsequently increased to 100% after the comprehensive CIA.48 The mean length of time supervised by the team was 10.6 weeks with an average of three CIA during the study period. Wound cultures were carried out for 100% of cases and results received for 85%. Out of the swabs received the following isolates were found: Pseudomonas aeruginosa: 8% (n=1), Proteus mirabilis: 15% (n=2) mixed gram negative bacteria: 8% (n=1), Group B streptococcus: 8% (n=1), commensal flora: 46% (n=6). The swabs were taken at the first visit; the validated NERDS and STONEES tool was used to clinically identify the presence of infection and antibiotic therapy was prescribed accordingly (systemic antibiotics for deep and surrounding infection; topical antimicrobials for superficial infection). The majority of the cohort (n=9, 69%) had deep and surrounding tissue infection (≥3 NERDS and STONEES criteria). Antibiotics were prescribed for all patients (n=13) based on evidence practice (broad spectrum and anti-inflammatory antibiotics) to address both the infection and inflammatory components of HS. Cotrimoxazole, doxycycline, and metronidazole were the most commonly used antibiotics (n=7, 54%; n=6, 46%; and n=5, 38%, respectively). Most patients were given a combination antibiotic therapy (n=7, 54%). Topical clindamycin with benzoyl peroxide was most commonly used (n=11, 85%), followed by injection of intralesional steroids (n=6, 46%). Dressing regimen was changed at CIA for a majority of the cohort (n=9, 69%). Neither intravenous antibiotic therapy nor biologics were prescribed for the cohort. Most of the visits required acute minor surgical interventions (incision of abscesses and removal of amorphous material from the base or surface curette of sinuses). Other treatments used were: silver sulfadiazine cream, hydrogel, hexachlorophene detergent cleanser, foam with detergent, and silver action exudate absorptive fabric with silver (Table 2).
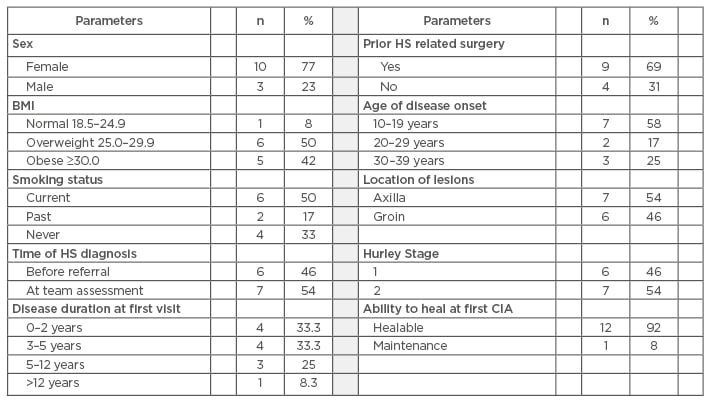
Table 2: Results of a retrospective chart review.
HS: hidradenitis suppurativa; CIA: comprehensive interprofessional assessment.
At the first comprehensive assessment 38% (n=5) were discharged from the clinic. At the 4-week follow-up visit 15% (n=2) had an increase in wound size, 23% (n=3) had a 30% decrease in wound size, and in 15% (n=2) the size could not be measured or was not measurable (in cases such as nodules or sinus tracts).
Patient-Centred Concerns
Patient-centred concerns reported by the cohort included impairment of activities of daily living (n=7, 54%), body image compromise (n=6, 46%), and inability to participate in sports and exercise (n=2, 15%). Mean pain score on assessment was reported to be 3.4 out of 10 on the numeric pain scale. No specific tool was used to determine quality of life (general wellbeing, decreased pain levels, increased mobility, improved appetite) since their first visit. They were asked to report if their quality of life had improved, worsened, or was unchanged. Out of the 8 patients that had a second visit, 62% (n=5) reported an improvement in quality of life while 38% (n=3) reported it to be unchanged.
DISCUSSION
The results from our cohort study are largely consistent with findings in the literature. The majority of patients were females (sex ratio ˜3:1) and most had experienced onset of symptoms in their early 20s. Smoking and obesity were also found to be consistently higher than the general population, supporting their role as HS risk factors.19,21-25 Smoking cessation, dietitian, and exercise consultations were included in the treatment plan, as recommended by the European Guidelines for management of HS.41 Other investigational tests were ordered to determine underlying or undiagnosed comorbidities (diabetes mellitus and Crohn’s disease).8,25,27,31,50 Diabetes mellitus was newly diagnosed for one patient of the cohort.
Less than half of the patients (n=6, 46%) had a diagnosis of HS prior to their referral for an interprofessional assessment. Self-reported data from the cohort revealed that these patients had numerous prior surgical interventions, had previously been on multiple short doses of antibiotics, had high pain scores, and required frequent medical attention. This translates to higher healthcare costs as shown in a 2014 North American study that identified the major cost for patients with HS was inpatient costs and more frequent emergency department visits.33,35 The mean time from onset of symptoms to diagnosis was 4.2 years, ranging from 1–7 years, which is in line with findings from other studies.11,31,34 The total length of time with HS ranged from 3–10 years, consistent with the chronic forms of this condition. Patients within the cohort had been diagnosed with either Hurley Stage 1 or 2 by the interprofessional team, therefore these results do not accurately represent the distribution of Hurley Stage 3. This could be attributed to the fact that this clinic serves a specific geographical area of the general population of patients on homecare. These patients were primarily treated for abscesses, chronic wounds, and other diagnoses with the primary diagnosis of HS being missed. In addition, those patients that were accurately diagnosed with Hurley Stage 3 at the primary care level were probably being followed by general and plastic surgeons and not referred to the clinic.
Bacterial damage was not accurately identified at the community level when compared to the interprofessional assessment. The majority of the cohort was diagnosed with deep and surrounding infection utilising a validated tool by the interprofessional team.49 Secondary infection is not common in HS in general. However, in this population, the majority of patients in the cohort had chronic wounds, were undiagnosed with HS, and were not treated appropriately for infection or optimally for the inflammatory component of HS at the time of consultation. All patients were treated with systemic antibiotics: mainly combination therapy to address both the inflammatory and infection components of HS.34,37,41,44 Biologics were not required in this patient population, but after treating deep and surrounding infection and optimising local wound care, if deep inflammation persists, patients may benefit from adalimumab or other TNF inhibitors. Surgical management was introduced to the majority of patients prior to referral. Some clinicians favour local surgery for Hurley Stage 2 patients and more extensive surgery for Hurley Stage 3 disease, which should be introduced early in treatment, as outlined in the European Guidelines by Zouboulis et al.5,41
A larger proportion reported that their day-to-day activities were affected, mainly due to decreased mobility and pain, followed by embarrassment and body image issues. These findings are also consistent with the literature29,30,32,50 and a Canadian study that identified a profound effect on patients’ lives (DLQI score of 10±8.8), which is higher than for any other comparative chronic dermatological disorder.33 At the first follow-up interprofessional assessment after adequate pain management,51 the mean pain score was decreased by >50%.
The interprofessional assessment of these patients allowed for improvements in wound parameters and management. After the first interprofessional assessment, 38% of the patients were discharged from the study and followed-up by a dermatologist. Approximately one-third of the patients (38%) had complete wound closure by the end of the study period.
Utilising the concept of the wound bed preparation paradigm, treating the underlying cause including bacterial damage and persistent inflammation, addressing patient-centred concerns, and appropriate local wound care were the key management strategies to achieve better outcomes.48
STUDY LIMITATIONS
Study limitations include the small sample size, selection bias, and short follow-up time with difficulties in determining remissions and morbidity.
CONCLUSION
This study corroborates most recorded literature findings. Our cohort was predominantly female, disease onset was typically in the mid-20s, and had a high impact on activities of daily living with a delay in diagnosis. This study was conducted on a difficult-to-treat Canadian population not responding to usual treatment and highlights the difficulty of diagnosing HS early. The effectiveness of an interprofessional team approach, leading to earlier HS diagnosis when compared to standard community care was demonstrated. Key strategies to consider are developing the expertise and diagnostic acumen of primary care physicians and other community providers for persons with HS. A co-ordinated process of care with primary care physicians and interprofessional teams or HS centres of excellence may decrease healthcare costs and improve other health indicators.







