Interview Summary
Melanoma of the skin is a common type of cancer that primarily affects younger patients. In Stage III melanoma, which does not involve distant metastases, radical resection is curative in 40–50% of the cases. Adjuvant therapy should be discussed with suitable patients, as it can decrease the chance of, or extend the time to, relapse. Such therapies include mitogen-activated protein kinase (MEK), B-Raf proto-oncogene (BRAF), or serine/threonine kinase inhibitors, and immunotherapies against programmed death 1 (PD-1). These agents have significantly improved relapse-free survival (RFS) rates compared with placebo. However, adverse events (AE) associated with these treatments, although predominantly treatable at the time, may have longer-term consequences in some cases, including as yet unknown impacts on fertility. Three experts in the field of melanoma discussed with EMJ some of the issues around utilising adjuvant therapies for patients with resected Stage III disease. They highlighted the importance of including in the initial patient consultation not only information on survival outcomes, but also potential AEs, practical considerations regarding therapy choice, the impact of therapy on quality of life (QoL), and the possible need for cryopreservation, given the potential impact of these therapies on fertility. The experts also discussed the need to develop biomarkers that could help identify which patients may derive most benefit from adjuvant therapy, and those more likely to experience AEs. Awareness of both the advantages of adjuvant therapy, and short- and long-term impacts on health-related QoL (HRQoL), can help when discussing therapy choice with a patient.INTRODUCTION
In the USA and the UK,1,2 melanoma of the skin is the fifth most common type of cancer. American Joint Committee on Cancer (AJCC) staging of cutaneous melanoma is based on categories of primary tumour thickness (T) and ulceration; presence of loco-regional lymph node metastases (N); and presence of distant metastasis (M) to both skin and other organs. Stage III subgroups, which are the focus of this expert discussion, range from IIIA to IIID, and include, according to AJCC, diagnoses of any subtype of melanoma with at least one lymph node involved, and no distant metastases.3
Kaplan–Meier melanoma-specific survival rates for Stage III melanoma are estimated to be between 32−88% at 5 years, and 24−88% at 10 years, depending on A−D subgroup, which are delineated by tumour (T0: no primary tumour, to T4b: tumour >4 mm with ulceration) and node (N1a: one tumour-involved node, to N3c: ≥2 clinically occult or clinically detected nodes and/or any number of matted nodes).3 The recent improvement in survival rates is most likely due to the use of adjuvant therapies (Figure 1).4-6
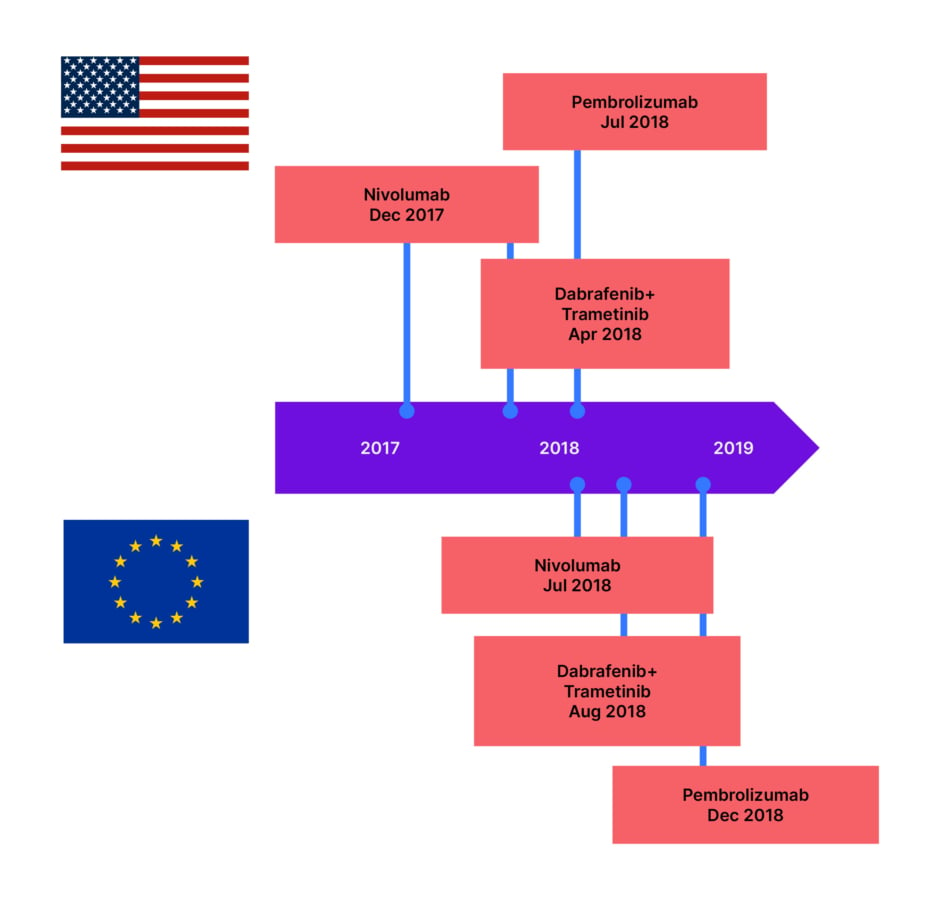
Figure 1: Targeted therapies and immunotherapies approved for Stage III resected melanoma.7-11
To help to understand some of the key considerations for use of these therapies, EMJ discussed a variety of issues related to adjuvant melanoma treatments with three physician researchers: Teresa Amaral, Skin Cancer Clinical Trials Center, Eberhard Karls University of Tübingen, Germany; Mario Mandalà, Università degli Studi di Perugia, Italy; and Axel Hauschild, University Hospital Schleswig-Holstein, Kiel, Germany. These experts all have involvement in clinical trials and/or real-world investigations of adjuvant treatment for resected Stage III melanoma.
Adjuvant treatments that may be utilised in this setting include a combination of the orally-administered targeted therapy trametinib, an MEK inhibitor,11 and the BRAF inhibitor dabrafenib,10 indicated for patients with BRAF V600-mutant melanoma. This combination was investigated in the Phase III COMBI-AD trial (NCT01682083),12 where patients with Stage III melanoma were treated with dabrafenib+trametinib combined (n=438), or placebo (n=432). At 5 years, disease relapse or death before relapse occurred in 43% of the combination group, compared with 61% of the placebo group. The hazard ratio (HR) for RFS with the combination treatment compared with placebo was 0.51 (95% confidence interval [CI]: 0.42–0.61).4
Adjuvant therapies for melanoma also include intravenous (IV) therapy with checkpoint inhibitors against PD-1 (including pembrolizumab and nivolumab).8,9,13,14 In the Phase III KeyNote-054 trial of pembrolizumab versus placebo (NCT02362594),15 the 5-year RFS HR was 0.61 (95% CI: 0.51–0.72).5 Indirect treatment comparison of these results with those of the Phase III CheckMate 238 trial, which investigated nivolumab versus ipilimumab for Stage III/IV resected melanoma (NCT02388906; n=453),6,16 and the Phase III European Organisation for Research and Treatment of Cancer (EORTC) 18071 trial, which compared ipilimumab to a placebo (NCT00636168),17,18 showed that 4-year RFS for nivolumab had a HR of 0.49 (95% CI: 0.39–0.61), with a RFS HR of 0.69 (95% CI: 0.56–0.85) for ipilimumab compared with nivolumab.19
With these figures in mind, the first discussion point focused on treatment choice in the adjuvant setting. Mandalà explained how, “if the risk is minimum, for example, a patient with Stage IIIA melanoma and microscopic lymph node involvement with a tumour burden less than 1 mm, we need to discuss not the relative risk reduction, but the absolute risk reduction, and the number needed to be treated to see benefits with adjuvant therapy.” He added: “This is important for all patients, not only for Stage IIIA disease. For patients with Stage IIIA and <1 mm tumour burden in the node, it is important to consider the risk benefit, since these patients have at baseline a good prognosis.” Mandalà also discussed how: “If we consider a patient with high tumour burden, palpable nodes, or very thick, ulcerated melanoma, with several local lymph nodes involved, the risk for short-term recurrence is more preserved with targeted therapy if the melanoma harbours a BRAF mutation.”
‘SURVIVAL’ IN ADJUVANT THERAPY TRIALS
One aspect of note in adjuvant therapy trials is that the primary endpoint used is RFS, defined as ‘time from treatment start to disease recurrence or death from any cause’.20 Endpoints for clinical trials investigating cancer therapies may also include overall survival (OS; time from therapy start or diagnosis until death from any cause), which is considered the ‘gold standard’ endpoint; progression-free survival; disease-free survival; and disease-specific survival.21 However, as Amaral discussed, “in some cases, the time required to obtain OS data may be quite long, and this may not be available for the discussion about adjuvant therapy. Without OS data available, and if after the requested follow-up there is no OS benefit, even if the patients have a long time without disease recurrence, i.e., prolonged RFS, it might be that the time they will live in total will be the same, whether they receive treatment or not.”
Due to OS not yet being known with newer adjuvant therapies, Amaral explained that “we need to have earlier data to see how, [for example] distant metastases-free survival, event-free survival, or RFS are impacted by the treatments we are giving.21 Event-free survival and disease-free survival,” she continued, “are acceptable endpoints for most of the regulatory agencies, in terms of approval in the adjuvant setting.” Hauschild additionally explained how “agencies [also] accept RFS as the endpoint, which I am happy with, because this means the approval of drugs earlier for patients.” This may be an important discussion point with patients, as, Hauschild added, “we know that relapse is a landmark in the patient’s life, it is a really terrible event.” Amaral also emphasised how it is important to talk to the patient, and make them aware of available clinical data for the therapy they could be prescribed, as “for the patients, it is quite important to know how long they will be disease-free, because in the end this is the time they will not need to receive treatment; in terms of QoL, that is an important aspect.”
WHAT PATIENTS RATE AS IMPORTANT WHEN CONSIDERING TREATMENT
According to Amaral, following surgery for Stage III melanoma, “patients are, in principle, tumour-free, so their main concern is whether they will benefit from adding adjuvant treatment in terms of survival.” “It is important to see the patient’s side of the discussion,” said Hauschild, “and not the physician’s side.” With this in mind, Mandalà noted that, “what we have learned in the last 10 years is that patients’ want is to live after their cancer diagnosis, to be cured, to have a life perspective, to build something: family, dreams, hopes.” However, Mandalà further explained how “what they ask first is to spare recurrences; after this, they ask to live without long-term sequalae or toxicities.” This was confirmed in the COMBI-AD trial of adjuvant dabrafenib+trametinib, where patients rated their most important concern as prevention of tumour recurrence. This was ranked above concerns regarding AEs with treatment.22
In a ‘discrete choice experiment’ of 116 people with Stage III resected melanoma who were candidates to receive adjuvant immunotherapy, patients selected such treatment in 70% of the scenarios offered. Choice was swayed by reductions in the probability of recurrence, but also by reductions in the probability of permanent or fatal AEs, as well as by costs.23 “These are important aspects to consider and put on the table when we speak with the patient about the pros and cons of treatments,” explained Mandalà. “If the efficacy is similar with two treatments, it is important that patients consider, and are aware and informed, regarding the long-term tolerability and AEs related to adjuvant therapy.”
While adjuvant therapy may benefit a patient, Hauschild discussed how it was equally important to understand why a patient may not choose this type of therapy. This was investigated in a German survey including patients with Stage III–IV melanoma without evidence of disease following tumour resection, but with an indication for adjuvant therapy. Of 904 patients, 23.1% did not opt for such therapy, with higher rates of rejection in patients who were older, had more comorbidities, and had a lower tumour stage.24 “The third point is very important,” said Hauschild, “Stage IIIA patients were more often than Stage IIIB–IIID patients to say no to adjuvant treatment, and I believe it is not because the patients know everything about the disease, but is because patients were explained that in Stage IIIA, with low tumour burden in the central node, the risk for relapse is substantially lower than for any other patient. So, if the physician explains to a patient that the risk of relapse is 10% or 15%, [the patient] responds differently to the question of adjuvant treatment than a patient where there is a 70% risk of relapse.”
Reasons given for rejecting adjuvant therapy in the German survey included treatment inconvenience, low risk of recurrence (in the patient’s opinion), QoL factors, and fear of AEs.24 Hauschild commented: “Most patients ask, can I still continue with my work? Can I still continue with my daily routine? Is this affecting my family life? It highly depends on how you explain [these factors] to patients, whether they say yes or no to the treatment modalities, because they might also have fear to die from the disease. We need to outweigh the fear to die with the fear of AEs, and loss of QoL, which is very much dependent on the explanations of the treating physician.”
Amaral also outlined how treatment choice has a number of practical aspects, so the physician needs to explain what a patient’s choice will imply “in terms of how many times they need to come to the hospital. Most of these patients are professionally active. They need to know how long they might stay out of work, and if this will have an impact on their QoL. For those that have a BRAF mutation, the fact that they can receive either oral therapy or IV therapy might weight the treatment decision in some cases. For some patients, it is difficult to travel to receive IV therapy,” remarked Amaral, “and we have patients that do not want to receive IV therapy; therefore, oral therapy is definitely an alternative in terms of administration.”
Practical issues that can influence a patient’s treatment decision include, according to Amaral, “if they need to come every 3 or 6 weeks to the hospital to receive IV therapy, depending how far away they are; if they are working abroad and therefore cannot afford to come to us so often; or if they have access to a general practitioner that can treat AEs.” For some patients, explained Amaral, “if they are candidates for targeted therapy, they will opt for it because it is more practical for them. The patients have the medication at home, and while in the beginning they still need to come to the hospital [for laboratory controls and toxicity evaluation], one of the biggest advantages is that, even if the patient is travelling, for example, they can get a prescription for a longer time.” This gives the patient, added Amaral, “freedom to decide when they need to come to us or not [if the patient does not have any AEs] and empowers the patients with some flexibility.”
POST-RESECTION TREATMENT-RELATED ISSUES FACED BY PATIENTS WITH MELANOMA
According to Mandalà, “patients in the adjuvant setting are a specific population with peculiar characteristics. As these are patients that may be cured by surgery, if they receive an adjuvant preventive treatment to reduce the risk of recurrence, the AEs are very important to consider. First, the acute AEs, but also the long-term and chronic AEs, and how these AEs can impact on QoL.” Mandalà discussed how, “if we explain in detail the pros and cons of both treatments, the patient can decide based on practical considerations. We have the same long-term benefit with targeted therapy and immunotherapy, but what makes a difference is the long-term toxicity with one treatment versus the other. In clinical practice, my experience is that, if well-explained, if we have an open conversation with our patients, and give them space and time to consider one treatment versus the other, they can make a choice.”
As AEs differ depending on the type of treatment, Amaral emphasised that “this is one of the things that is a decision point for the patient.” For example, in the CheckMate 238 trial of nivolumab, acute (0–3 months) on-treatment AEs included pruritus (15.5%), diarrhoea (15.3%), skin conditions (0.2–11.7%), hypothyroidism (5.3%), liver enzyme increases (3.1–7.1%), and hyperthyroidism (2.9%). These were mostly Grades I–II (67.7%), with 4.6% Grades III–IV. The majority of AEs lessened between 3–12 months of nivolumab treatment, with the most prominent in this period being rash (7.5%), diarrhoea (7.3%), pruritus (7.3%), and hypothyroidism (5.5%).25 With immunotherapy, explained Amaral, “most frequent are AEs that we can treat with immunosuppression, mostly with cortisone.” Such treatment was administered to 31.0% of the patients in the CheckMate 238 trial.25 Following treatment for AEs, Amaral explained how, “in most of the cases, we can restart the treatment if needed, and if the patient agrees.”
“In terms of targeted therapy,” continued Amaral, “the profile of AEs is quite different.” In the COMBI-AD trial of dabrafenib+trametinib, or placebo, the most common AEs during treatment were pyrexia (any grade: 63% versus 11%; Grade III−IV: 5% versus <1%), and fatigue (any grade: 47% versus 28%; Grade III–IV: 4% versus <1%). Other AEs (any grade) were nausea (40% versus 20%), headache (39% versus 24%), chills (37% versus 4%), diarrhoea (33% versus 15%), vomiting (28% versus 10%), arthralgia (28% versus 14%), and rash (24% versus 11%). For all these, Grade III–IV AEs only occurred in 0–1% of patients. AEs leading to discontinuation occurred in 26% of patients in the combination group, compared to 3% of patients in the placebo group.26
In these trials, pyrexia was the most common AE,26,27 and Amaral described how this is most often treatable with antipyretic therapy and treatment holidays, and how, overall, most of the AEs are “short-living.” She also explained how, “in most of the cases, the symptoms and complaints disappear once we’ve stopped treatment for a couple of days, and then we can restart it, depending on the grade.” However, Amaral also cautioned how AEs may reappear once treatment is restarted, even with dose reduction, and “in some cases, we need to stop treatment for quite some time, or even permanently discontinue it.”
Hauschild confirmed that “this reflects my personal experience,” but also described how treatment discontinuation “in real life is lower, because we have different rules for stopping drugs, and for treatment holidays, which can maintain QoL.” Mandalà discussed how pyrexia associated with targeted therapy can be reduced by employing a modified algorithm of treatment management. This approach was investigated in the COMBI-APlus trial (NCT03551626),28 where both dabrafenib and trametinib were automatically discontinued in patients with pyrexia ≥38 oC, then resumed once pyrexia had abated for ≥24 hours. This regimen reduced pyrexia-related toxicities and events, such as hospitalisation and permanent treatment discontinuation, compared to that shown in the COMBI-AD trial, where only dabrafenib was stopped, and only when pyrexia reached ≥38.5 oC.29,30 While in COMBI-AD, the incidence of composite pyrexia events (Grade III–IV pyrexia, hospitalisation, or permanent discontinuation due to pyrexia) was 20.0% (95% CI: 16.3–24.1%),31 in COMBI-APlus it was only 8.0% (95% CI: 5.9–10.6%).30
Because of the potential for these AEs, remarked Amaral, “one of the things we tell patients is that it is better to report more than less AEs. If they are unsure whether this is an AE, they should report it, because it is easier to deal with an AE earlier than later on, when it is already fully established. This kind of support and information in the beginning can minimise some of the long-term issues.” Amaral also noted that, with regard to AEs, “another very important aspect is the time invested in teaching and informing other stakeholders that see the patient, not only in reference centres, but also general practitioners or nurses that take care of them near their home, or in the primary care environment.”
POTENTIAL LONGER- TERM TREATMENT-RELATED ISSUES FACED BY PATIENTS WITH MELANOMA AFTER ADJUVANT THERAPY
With regard to longer-term AEs associated with adjuvant therapy, Mandalà discussed how “there is an underestimation of such effects by clinicians as, [in general], long-term effects have not been reported in clinical trials.” He explained how this may be because “one of the main problems is that we collect toxicities within 100 days after the end of treatment, so we do not have any focused idea of what happens afterwards.” However, Mandalà continued, “this is an important issue, because there is a life after melanoma diagnosis, and we should consider these long-term and potentially impactful [AEs] in daily life.”
Analysis of data from the CheckMate 238 trial of nivolumab showed that, from first dose to 100 days after the last dose, 11.3% of 452 patients reported first occurrence of hypothyroidism, 8.0% of hyperthyroidism, 2.0% of thyroiditis, 1.8% of hypophysitis, 1.3% of adrenal insufficiency, and 0.4% of diabetes.25 Mandalà therefore stressed the need “to follow patients to identify chronic AEs or severe AEs as, in some cases, some AEs do not occur during treatment, and can occur after stopping the treatment.” For example, following anti-PD1 therapy, one study of 118 patients found that the estimated incidence of a delayed immune-related AE (irAE) was 5.3% (95% CI: 4.0–6.9.), with median time to occurrence of 16 months (range: 12–53 months). The most common types of irAEs were colitis, rash, and pneumonitis, with these being ≥Grade III in 39% of patients. In this study, 69% of patients required steroids, and 23% required additional immunosuppression. There were also two deaths in this study related to irAEs, one of which occurred 11 months following therapy cessation.32 Another study of adjuvant anti-PD-1 therapy investigated the chronicity of irAEs. Those that persisted beyond 12 weeks following therapy discontinuation (found in 43.2% of 387 patients) included endocrinopathies, arthritis, xerostomia, and neurotoxicity. Of note though, most of these irAEs (96.4%) were mild.33
Another potential long-term AE, which Mandalà showed in a case study following use of an immune checkpoint inhibitor, is the ‘memory effect’, where, he explained, “there is a memory against the tumour and against normal tissues.” Diffuse bilateral lung lesions with alveolar opacities and nodules were observed in this case, which Mandalà suggested were due to inflammatory and autoimmune-related issues.34 As such, Mandalà described how, “during the long-term, we generally evaluate and visit patients every 4 months during the first 2 years after stopping treatment, and they can call in case of issues.” He also noted how, “with immunotherapy, we need to create a multidisciplinary approach, because we need to have specialists that can address specific issues related to autoimmune disease that can emerge during treatment, or after stopping it.”
Mandalà emphasised that “persistent life-altering, life-threatening AEs should be better characterised in detail. These figures should also be integrated into patient counselling and treatment decision-making, because this is important to discuss where we consider one treatment versus another treatment in the adjuvant setting. Generally, we consider only severe or life-threatening toxicity, but there is background and Grade II chronic toxicities that are even more important, because this happens in hundreds of patients treated with adjuvant therapy, and these have an impact on their daily life.”
However, while these figures may seem large, according to Hauschild, “we only have a small number of patients who may suffer from serious autoimmunities that are irreversible, and may need hormone replacement for the rest of the life.” For example, he said, “thyroiditis may lead to replacement of thyroxine and, for patients who are suffering from hypophysitis, [they need] cortical steroid hormone replacement plus thyroid hormones. This can affect their QoL.”
“On the other hand, with the MEK and BRAF-directed treatment modalities,” Hauschild explained how, in general, “we know there are no long-term sequelae, no long-term AEs.” However, he did describe rare exceptions to this, and added: “In all the years where I used BRAF and MEK inhibitors in clinical trials, and after approval, I have had only one patient with a long-term AE, a 26-year-old lady who got uveitis, and is night blind, which is impacting her QoL, as she cannot drive a car during the night.”
QUALITY OF LIFE IN A PATIENT WHO HAS SURVIVED MELANOMA
“Normally, as clinicians, we are primarily focused on survival advantage,” said Amaral. “However, QoL is a very important aspect.” “The QoL question is very important in the adjuvant setting,” agreed Hauschild, adding: “The lower the disease stages are, the better QoL needs to be, because we overtreat more patients in Stage IIIA than in Stage IIID. This means that we need to have a drug that is well tolerated, does not lead to long-term sequelae, and that QoL should be at least balanced between the placebo arm and the treatment arm.”
Of note, in the COMBI-AD study, change from baseline in several HRQoL scores was not significant or clinically meaningful in patients treated with a dabrafenib+trametinib combination, or a placebo regimen, either at the time of treatment, or during long-term follow-up.22 Similar results regarding HRQoL were found in long-term follow-up of the EORTC 1325-MG/KeyNote-054 study (NCT02362594)15 of pembrolizumab or placebo,35 and in the CheckMate 238 trial of nivolumab.36
Hauschild also described that at their centre, “regarding long-term QoL during and after treatment, there is a structured programme of low-to-moderate levels of exercise for cancer patients. This impacts particularly fatigue during treatment, and helps to maintain QoL.”
THE ROLE OF BIOMARKERS IN TRIALS OF ADJUVANT THERAPY
In clinical practice, AJCC staging is the predominant way in which melanoma is categorised,3 and according to Hauschild, this means that all Stage III patients, whether Stage IIIA or Stage IIID, “have the opportunity to be treated.” However, explained Mandalà, “there are some weaknesses related to the AJCC classification because in the adjuvant phase, we have overtreatment of patients, as some are completely cured by surgery and do not need therapy.” Hauschild agreed, estimating that “we are overtreating roughly 70% of patients in Stage IIIA, whereas we are only overtreating [an estimated] 30% of patients in Stage IIID.”
With these figures in mind, Mandalà discussed how “we need to be more precise, by introducing biomarkers to identify and focus on patients with high risk of recurrence, to increase the absolute benefit, and to spare some patients adjuvant therapy.” Hauschild further added that “we would love to have a decision-making factor for saying ‘this patient needs a treatment, the others do not need the treatment.” He explained how biomarkers also need to be able to inform a patient “that you are safe if you receive immune checkpoint inhibitors, or you are not a good candidate because you are more likely to suffer from these serious AEs.”
Biomarkers have been used in some clinical trials in the setting of adjuvant melanoma. For example, in CheckMate 238, it was found that RFS following either anti-PD-1 immunotherapy or targeted therapy was better in patients with lower levels of serum C-reactive protein, and higher levels of IFN-γ gene expression signature, tumour mutation burden, tumour PD ligand 1, and intratumoural CD8β T-cells. However, according to the study authors, these markers currently have ‘limited clinically meaningful predictive value’.37 Indeed, Hauschild commented that “patients who have low levels for the IFN-γ signature and a low tumour mutation burden can still have a good chance to benefit from adjuvant treatment, and therefore, we do not want to eliminate those patients who may need the drug.”
Another biomarker discussed by the experts was non-invasive examination of circulating tumour DNA (ctDNA), which has been investigated for patients with BRAF wild- type melanoma. Correlation has been found between high levels of ctDNA and any detectable ctDNA variants, and worse prognosis, with treatment response correlating with longitudinal ctDNA changes.38 Using this biomarker, said Hauschild, “may give us a clearer pattern of patients who need or who do not need an adjuvant treatment.” However, commented Mandalà, “one of the main problems is that […] we need to test and validate these biomarkers in prospective clinical trials to understand how impactful they can be in routine activity.”
THE IMPACT OF ADJUVANT THERAPY ON FERTILITY
“We need to recognise that melanoma is a disease of young people,” said Mandalà, “and impact on fertility is very important in both women and men.” However, he acknowledged, “the data we have are scarce, and we do not have enough evidence about the impact on fertility of immunotherapy and targeted therapy.” “What I think is very important for patients to whom we offer adjuvant treatment,” said Amaral, “is that they need to be aware that there might be an impact in terms of fertility, and that obviously women should not become pregnant and men should not father children while they are receiving treatment. Contraception needs to be discussed during and after treatment, with duration depending on the type of treatment they receive.”
Due to the currently unclear impact of adjuvant therapy on fertility, Amaral described how an important aspect when first consulting a patient regarding such treatment is discussion of cryopreservation of oocytes or ovarian tissue for females, and sperm for males. “Counselling upfront,” said Amaral, “or at least the possibility for the discussion, should be given when the patients are candidates for adjuvant therapy. With that information the patients can decide whether they want to do cryopreservation or not, depending on their family planning.” Amaral admitted that “these are aspects that sometimes may be left a little bit on the side, because we are very focused on the effects and potential benefits of adjuvant treatment, but in the end, if we have a very young patient or partner, we need to discuss this with them. They need to be aware that these issues might come in the future, and that there are ways to minimise the impact.”
Hauschild described how, in Germany, where cryopreservation is now paid for by public insurance, counselling patients when discussing potential fertility impacts with adjuvant treatment occurs “in interaction with a gynaecologist who is specialised in conservation of oocytes, as is done for gynaecological cancers.” To further elucidate the effects of adjuvant treatment on fertility, Mandalà described how “the Italian Melanoma Intergroup (IMI) is planning a prospective study to evaluate the impact of adjuvant therapy on patients with resected melanoma at high risk of relapse. This will include female patients under 40 years with Stage II−IV, completely resected melanoma, who are candidates to receive adjuvant therapy or immunotherapy. [There is also] a third cohort, just for observation, and a calibrator of what we observe in the targeted therapy or immunotherapy arms. The primary endpoint of this study is evaluation of serum anti-Müllerian hormone (a proxy of fertility).39 We want a longitudinal assessment before, during, and after stopping treatment, to look at the problem of fertility that needs to be addressed and answered, because this is a common question, particularly from young women who receive adjuvant therapy.”
CONCLUSION
Adjuvant therapies for Stage III melanoma have shown favourable outcomes in terms of RFS.4,5,19 However, experts highlighted that, given that patients can essentially be considered ‘tumour-free’ following surgical resection, the choice to use adjuvant therapy should also be based on impact on overall QoL with regard to short- and long-term AEs, fertility, and practical considerations, such as therapy administration. The use of biomarkers may, in the future, help guide treatment decisions as to which patients may benefit the most from adjuvant therapies, and the likelihood of experiencing potentially life-altering, long-term AEs.







