INTRODUCTION
This clinical dermatology report examines six short case studies of patients diagnosed with acne vulgaris, actinic keratosis (AK), and atopic dermatitis (AD). Demographics and clinical histories of each patient are provided, along with the symptoms and signs identified on initial and subsequent clinical assessments. Diagnosis, treatment, and follow-up evaluations of each patient are reported, and several images serve to illustrate the extent of the disease in each case. These images also support the therapeutic decisions made and are evidence of successful treatment management in patients with these distressing dermatological conditions.
ACNE VULGARIS
Acne is a common dermatology disorder affecting adolescents more frequently than adults or children, although acne in adolescent female patients may persist into, or even begin in, adulthood.1 Hallmarks of the disease are the characteristic presence of noninflammatory lesions (open and closed comedones) and inflammatory lesions (papules, pustules, and nodules), along with variable levels of scarring. It is important to recognise the psychosocial effects of acne on a patient’s quality of life, such as poor self-image, depression, and anxiety.2 Clinical assessments of available acne treatments should include the evaluation of local side effects such as erythema, which may occur in up to 70% of patients treated with topical retinoids. This heavily impacts on patients’ adherence to topical regimens, which is poor in approximately 50% of acne patients.3 Considering the multifactorial pathophysiology of acne vulgaris, various agents with complementary and synergistic mechanisms of action are generally combined in order to increase the efficacy of therapy and patient compliance.4 Combination therapy appears to be an effective and well-tolerated standard of acne treatment.3,4
Case Study One
A 24-year-old female presented with a number of lesions identified as open comedones (18), closed comedones (22), papules (17), and pustules (7). The patient complained of dry skin and irritation, and a diagnosis of moderate acne was made on acne assessment (Figure 1A, 1C). An onset of moderate inflammatory acne 5 years earlier had been treated with 0.1% adapalene gel once daily at bedtime over 8 weeks, as well as moisturisers once daily in the morning. However, there was a poor response to treatment and the patient refused to continue with the same therapy.
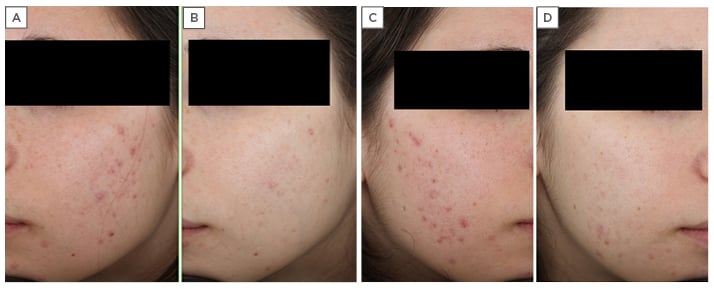
Figure 1: A 24-year-old female with moderate acne, resistant to previous treatments with 0.1% adapalene gel and moisturisers. A) Left side of face before treatment; B) left side of face after treatment with Acnatac® (clindamycin 1%/tretinoin 0.025%), showing clearing of lesions; C) right side of face before treatment; D) right side of face after treatment, showing complete clearing of inflammatory lesions and residual post-inflammatory hyperpigmentation.
Treatment
Acnatac® cream (clindamycin 1%/tretinoin 0.025%) was prescribed as a once-daily application at bedtime per 90 days. Acnatac is indicated for the topical treatment of acne vulgaris when comedones, papules, and pustules are present in patients aged ≥12 years.5
Post-treatment evaluation
Follow-up was scheduled for 30, 60, and 90 days. After 90 days there was complete clearing of inflammatory lesions with residual comedones and post-inflammatory hyperpigmentation (Figure 1B, 1D). At the end of treatment, the diagnosis was changed from ‘moderate’ to ‘mild’ acne with a reduction in the number of lesions: open comedones (8), closed comedones (10), papules (0), and pustules (0). A topical cosmetic keratolytic agent was prescribed for residual comedones.
Case Study Two
An 18-year-old female presented with a number of lesions identified on assessment as open comedones (13), closed comedones (16), papules (9), and pustules (4). The patient complained of dry skin, scaling, and irritation and, on assessment, a diagnosis of moderate acne was made (Figure 2A, 2C). There was an onset of mild inflammatory acne 3 years earlier which was treated with 0.05% tretinoin cream once daily for 5 months, along with moisturisers and the application of a sunscreen with a sun protection factor of 50+ once daily in the morning. As in case study one, this patient also had a poor response to her previous treatment, experiencing persistent background erythema, scaling, and stinging. The patient refused to continue with the previous therapy.
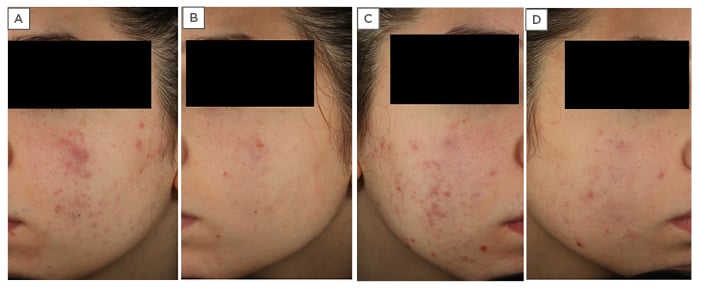
Figure 2: An 18-year-old female with moderate acne resistant to previous treatments with 0.05% tretinoin cream, moisturisers, and sunscreen with a sun protection factor 50+. A) Left side of face before treatment; B) left side of face after treatment with Acnatac® (clindamycin 1%/tretinoin 0.025%), showing clearing of lesions; C) right side of face before treatment; D) right side of face after treatment, showing ‘almost clearing’ of inflammatory lesions.
Treatment
Acnatac cream (clindamycin 1%/tretinoin 0.025%) was prescribed as a once daily application at bedtime per 90 days.
Post-treatment evaluation
Follow-up was scheduled for 30, 60, and 90 days and clinical features at the end of treatment revealed an ‘almost clearing’ of inflammatory lesions with excellent improvement in background erythema (Figure 2B, 2D). Acne severity at the end of treatment was diagnosed as ‘mild’ with a reduction in the number of lesions: open comedones (5), closed comedones (9), papules (1), and pustules (1). The patient was instructed to continue the treatment until lesion clearing.
Take-Home Messages
A dosing regimen of Acnatac cream (clindamycin 1%/tretinoin 0.025%) prescribed as a once daily application at bedtime per 90 days is a well-tolerated treatment for acne vulgaris, showing an effective reduction of background erythema and both noninflammatory and inflammatory lesions. Additionally, combination therapy is more convenient for patients than applying two separate formulations, supporting adherence with their treatment.
ACTINIC KERATOSIS
AK is characterised by chronic cutaneous lesions caused by chronic exposure to ultraviolet radiation.6 The prevalence of AK is known to increase with age and is most commonly seen in light-skinned populations;7 additional risk factors are male sex and cumulative sun exposure.6 AK lesions may be considered as a superficial squamous cell carcinoma (SCC) because they are similar to SCC at both the cellular and molecular level and have the potential to develop into invasive SCC.6,8
In addition to clinically visible AK lesions, subclinical areas of atypical keratinocytes occur in a field of chronic sun-damaged skin resulting in field cancerisation.6 As it is not possible to predict which lesions (clinical or subclinical) will transform to invasive disease, guidelines recommend that it is necessary to adequately treat all AK.6,9,10
Case Study Three
Evaluation and assessment of a 70-year-old male presenting with an itchy scalp revealed approximately 30 diffuse AK lesions (Figure 3A). Patient history included renal transplantation and previous treatments consisted of a single session of cryotherapy, two sessions of CO2 laser treatment, and multiple surgeries.
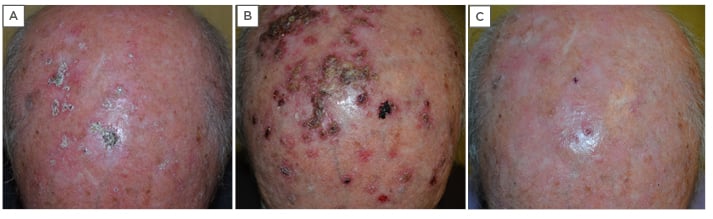
Figure 3: A 70-year-old male with diffuse actinic keratoses of the scalp. A) Before treatment with two cycles of Zyclara® cream (imiquimod 3.75%): one application daily for 2 weeks, followed by a 2-week rest period, then an additional 2 weeks of treatment; B) after the first cycle of 2 weeks of treatment, showing the presence of a diffuse inflammatory reaction as the result of field cancerisation; C) two weeks after the second cycle of treatment, showing an ‘almost clearing’ picture with few actinic keratosis lesions visible.
Treatment
The prescribed treatment was a once-daily application of Zyclara® cream (imiquimod 3.75%) before bedtime for 2 weeks, followed by a 2-week rest period, then another 2 weeks of treatment. Zyclara cream is indicated for the topical treatment of clinically typical, nonhyperkeratotic, nonhypertrophic, visible or palpable AK of the full face or balding scalp in immunocompetent adults when other topical treatment options are contraindicated or less appropriate.11
Post-treatment evaluation
The first follow-up was scheduled for 2 weeks after the first cycle and this revealed the presence of a diffuse inflammatory reaction as a result of the emergence of field cancerisation (subclinical) lesions as expected (Figure 3B). Clinical features on the second follow-up at 2 weeks after the second cycle revealed an ‘almost clearing’ picture with few AK lesions visible (Figure 3C). A single infiltrated lesion on the vertex of the scalp required further histological evaluation. According to the product label for Zyclara cream,11 the clinical outcome of therapy has to be determined after regeneration of the treated skin, approximately 8 weeks after the end of treatment, and on appropriate intervals thereafter based on clinical judgement. The final follow-up period took place 8 weeks after the end of treatment and showed no relapses. AldaraTM cream (imiquimod 5%) was suggested for residual isolated lesions.
Case Study Four
Evaluation and assessment of a 78-year-old male revealed approximately 30 diffuse AK lesions on the scalp, but the patient did not complain of any itching or burning linked with the lesions (Figure 4A). As a farmer, he had a history of chronic ultraviolet exposure, and previous treatments included two cycles each of field-directed photodynamic therapy and lesion-directed cryotherapy.
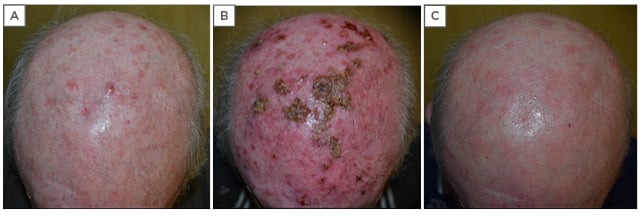
Figure 4: A 78-year-old male with diffuse actinic keratoses of the scalp. A) Before treatment with two cycles of Zyclara® cream (imiquimod 3.75%): one application daily for 2 weeks, then a 2-week rest period, then an additional 2 weeks of treatment; B) after the first cycle of 2 weeks of treatment, showing the presence of an intense inflammatory reaction with oozing and crusting, and diffuse erythema; C) two weeks after the second cycle of treatment, showing complete resolution.
Treatment
On this occasion, the prescribed treatment was a once daily application of Zyclara cream (imiquimod 3.75%) before bedtime for 2 weeks, a subsequent 2-week rest period, then another 2 weeks of treatment.
Post-treatment evaluation
Clinical features on the first follow-up at 2 weeks after the first cycle of treatment showed an intense inflammatory reaction with oozing and crusting; diffuse erythema was also observed as expected (Figure 4B). The second follow-up at 2 weeks after the second cycle revealed complete resolution (Figure 4C), and after 8 weeks no relapse was evident.
Take-Home Messages
The application of Zyclara cream (imiquimod 3.75%) to the skin of the affected field (area) once daily before bedtime for two treatment cycles of 2 weeks, each separated by a 2-week no-treatment cycle, is an effective therapy for multiple AK lesions and their cancerisation field. Zyclara cream can detect and clear both clinical and subclinical lesions and it can be used to treat large affected areas in one treatment course.9,10
ATOPIC DERMATITIS
In most countries, up to 20% of children and 2–8% of adults are affected by AD.12 This common disease, also known as atopic eczema, is a heterogenous skin disorder with a complex pathogenesis resulting from a possible combination of genetic and environmental factors. On establishing a diagnosis, treatment is based on disease severity using the Scoring of Atopic Dermatitis (SCORAD) method, where a score of >50 is indicative of a ‘severe’ condition, a score between 25 and 50 is considered a ‘moderate’ condition, and a ‘mild’ condition is indicated by a score of <25.12 Most cases are regarded as mild. Topical anti-inflammatories applied directly to the site of inflammation are central to the effective management of AD. The two predominant classes are topical corticosteroids (numerous different agents with differing potencies and formulations) and topical calcineurin inhibitors (pimecrolimus and tacrolimus). Topical calcineurin inhibitors offer a valid alternative as they have similar efficacy to low-to-mid potency topical corticosteroids, and are not associated with the same limitations such as skin barrier impairment and skin atrophy.13-15 Evidence has shown that pimecrolimus should be recommended as the treatment of choice for mild-to-moderate AD affecting sensitive skin areas (such as the face, neck, genital area, and inguinal folds).16
Case Study Five
A 16-year-old male complained of itching of the face and presented with typical AD lesions of the antecubital areas and face. Investigator Global Assessment (IGA) along with a Visual Analogue Scale (VAS) score (6) resulted in a diagnosis of mild disease with localised, erythematous desquamative lesions (Figure 5A). Personal and family history revealed that both the patient and his mother had previously been diagnosed with atopy (allergic rhinitis and asthma), which was treated with mid-potency topical corticosteroids for 3 weeks.
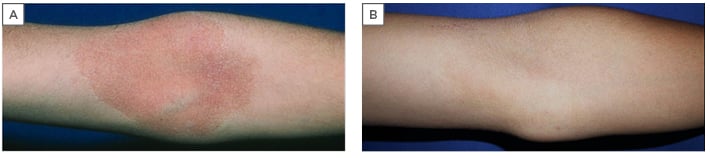
Figure 5: A 16-year-old male with typical atopic dermatitis lesion localised to the antecubital area, previously treated with topical corticosteroids with temporary results. A) Before treatment with Elidel® cream (pimecrolimus 1%) twice daily for 3 weeks; B) after treatment, with complete clearing.
This treatment had temporary results. The rationale for changing treatment, therefore, was the presence of localised patches and corticophobia.
Treatment
Elidel® cream (pimecrolimus 1%), which is indicated for mild-to-moderate AD, was prescribed twice daily for 3 weeks as recommended by the European Union (EU) guideline for children with facial lesions.12
Post-treatment evaluation
The first follow-up took place after 3 weeks of treatment: clinical features showed complete clearing at this point (Figure 5B). A second follow-up was made after an additional 2 weeks, where no relapse was seen.
Case Study Six
A 31-year-old female presented with clinical features of mild AD and lesions localised to the inner canthus of both eyes (Figure 6A–C). She also complained of some itching of the affected area (VAS score: 6). Family history revealed that her father had been diagnosed with AD that was treated occasionally with cosmeceutical creams.
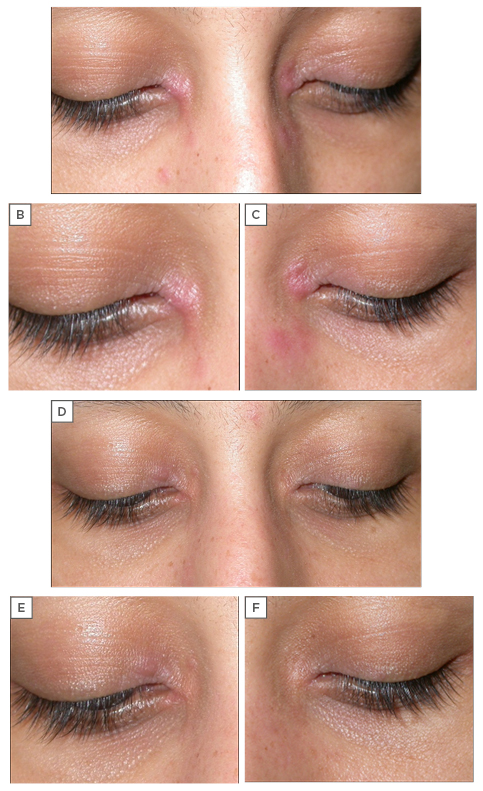
Figure 6: A 31-year-old female with atopic dermatitis, showing lesions localised to the inner canthus of both eyes. A) Before treatment with Elidel® cream (pimecrolimus 1%) twice daily for 3 weeks; B–C) details of the right and left eyes; D) after treatment, showing complete clearing of the lesions on the left eye and residual minimal inflammatory lesions on the right eye; E–F) details of the right and left eyes.
Treatment
The rationale for treatment in this patient was the presence of localised lesions in sensitive areas (periocular) and Elidel cream (pimecrolimus 1%) was prescribed for application twice daily for 3 weeks.
Post-treatment evaluation
The first follow-up was completed after 3 weeks of treatment and clinical features revealed a complete clearing of the lesions on the left eye and residual minimal inflammatory lesions on the right eye (Figure 6D–F). The second follow-up took place after an additional 3 weeks and showed no relapse.
Take-Home Message
A dosing regimen of Elidel cream (pimecrolimus 1%) indicated for mild-to-moderate AD and administered twice daily for 3 weeks is an effective and well-tolerated treatment for AD and can be safely used in sensitive areas such as the face and periocular areas.

![EMJ Dermatology 8 [Supplement 5]](https://www.emjreviews.com/wp-content/uploads/2020/07/ft-image-940x563.jpg)





