Abstract
Psoriasis is a chronic autoimmune skin disease with significant physical and psychiatric comorbidities. Research into psoriasis-associated depression has revealed several possible pathways linking the two very different diseases. Questions of causality arise when exploring the complex relationship of psoriasis and mood disorders, and studies have revealed that inflammation may serve as the common denominator linking psoriasis and depression. Conversely, many investigators have reported that psoriasis severity may fluctuate with perceived psychological distress, suggesting that psychological factors, rather than inflammation, may be the driving force behind disease exacerbation in these cases. The truth is likely a combination of both schools of thought: a bidirectional relationship between cutaneous and psychological disease manifestations with an overlapping biological mechanism associated with inflammation. Evidence has revealed multiple pathways by which this relationship can be explained, including hypothalamic–pituitary–adrenal axis hyperactivity, glucocorticoid receptor desensitisation, sympathetic nervous system activation, and altered expression of various chemical signals in the central nervous system. This review summarises the existing evidence and seeks to elucidate how the physiologic disturbances in psoriasis may contribute to both the cutaneous disease manifestations and associated psychological comorbidities. Evidence suggests that treating the psychiatric comorbidities of psoriasis can significantly improve cutaneous disease severity and treating the underlying inflammation could have profound effects on psychological health and quality of life. Therefore, conceptualising psoriasis as more than a purely dermatologic disease is useful in formulating a comprehensive treatment plan; furthermore, addressing both the cutaneous and psychological facets of this disease could prove profoundly beneficial for decreasing the associated negative impacts on patient quality of life.
INTRODUCTION
Psoriasis is a chronic autoimmune skin disorder that affects 2–3% of people worldwide.1,2 While the exact pathological mechanism is not fully understood, the disease seems to be associated with increased activation of cutaneous T cells, macrophages, and dendritic cells.3 This is followed by an increase in proinflammatory cytokines such as IL-1, IL-6, IL-12, and TNF-α, which induce a more generalised inflammatory reaction.4 Simultaneously, IL-6 and IL-23 activate Th17 cells, resulting in the subsequent release of IL-17, a potent stimulator of keratinocyte proliferation.5 While we have seen dramatic advancements in psoriasis treatments in recent decades, there is still significant room for improvement in addressing the disabilities associated with this condition.6
Psoriasis confers levels of disability that rival many major diseases, such as chronic heart failure, chronic obstructive pulmonary disease, and cancer.7 Psoriatic lesions can be physically disabling, especially if on the hands or feet,8 and patients with psoriasis are also at increased risk of cardiovascular disease, diabetes, psoriatic arthritis, and inflammatory bowel disease.9 Psychiatric comorbidities also have significant prevalence, estimated to affect at least 30% of patients with chronic dermatologic disease.10 Psoriasis specifically has been associated with a 39% increase in depression, 31% increase in anxiety, and 44% higher rate of suicidality compared with the general population.11 In addition, more severe clinical presentations have been associated with a 72% higher prevalence of depression when compared to milder disease.11 This difference is seen consistently in epidemiologic studies, even when controlling for possible confounders such as age, sex, race, weight, medical comorbidities, and drug use.12 Patients with severe psoriasis have also been found to be 69% more likely to attempt suicide13 and 30% more likely to complete suicide than those with milder forms of the disease.14 While one may reasonably assume that mood disorders experienced by patients with psoriasis are likely to be the consequence of social anxiety and stigma resulting from the disfigurement of their disease, this may not be the entire explanation. Psoriasis patients have significantly higher rates of depression and anxiety when compared to patients with skin disorders that are equally as disfiguring.15 This finding has spurred investigations to determine the possible existence of physiologic factors underlying both psoriasis and psychiatric disease.
A parallel to the classic chicken or the egg argument arises when pondering the intricate relationship between psoriasis and psychological distress. Inflammation in psoriasis may cause or worsen symptoms of mood disorders;16 however, the contrary may also be true, with some studies claiming that psychological distress may cause worsening disease severity in psoriasis patients, an effect that likely extends beyond the simple notion that depression can negatively impact compliance to psoriatic treatments.17,18 The truth is likely more complex, involving a bidirectional positive feedback loop that can drive and propagate a cycle of worsening depression and inflammation.19 In either case, patient quality of life is affected as a consequence of the psychological aspect of their disease, and there is a significant need for physicians to be able to treat psoriasis as more than solely a dermatologic condition.
PSYCHOLOGICAL DISTRESS CAUSING INFLAMMATION
There is ample evidence suggesting a strong link between depression and inflammation, particularly the Th1-like cell-mediated immune response.19,20 Psychological stress and trauma are two of the most well-studied risk factors of depression and have also proven to be risk factors for inflammation.21 In animal models, stress and trauma can increase inflammatory markers such as IL-1 and IL-6 in the brain, and these increases have correlated with greater depressive symptoms.22-25
Learned helplessness occurs when an animal endures recurrent stress or trauma that they have no control over, and repeated failures ultimately cause the animal to stop attempting to escape or alter their circumstances. In one common laboratory scenario, rats are repeatedly subjected to electrical shock through the floor of their cages. While initial trials evoke an escape response, as evidenced by a frantic rat clawing at its cage, this natural response is ultimately extinguished and subsequent shocks produce no apparent desire to flee, even if the cage door is opened. Learned helplessness has shown to be an effective simulation of depression and anxiety when testing the effectiveness of antidepressants in animals.21,26 Blocking IL-1 receptors in rats interfered with their ability to express fear and learned helplessness;23 this suggests that an inflammatory response in the brain might be necessary to enable full expression of depressive symptoms. Similar studies in humans have shown increased levels of IL-1β, IL-6, and TNF-α in response to mild emotional stress, which has led many to conclude that distress and depression are, at least in part, inflammatory reactions.27,28 This may have implications in the setting of psoriasis, as several studies have linked these inflammatory markers with psoriatic plaque formation.29 There have been great strides in this field but more research is needed before we can fully understand the complexities of the relationship between the mind and the immune system.
There is accumulating evidence that defines the skin as an extension of the neuroendocrine system, which is capable of responding to central hormone levels as well as controlling neuroendocrine environments locally.30 A pathway linking psychiatric stress to an inflammatory immune response would have to activate cell-mediated autoimmunity and trigger the chronic inflammation seen in psoriasis patients. One possible mechanism that satisfies these criteria may involve hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, which is commonly seen in depression.31 HPA axis hyperactivity results in the release of significantly more corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and cortisol compared to unaffected individuals.32 The potential role of CRH in psoriasis is supported by the discovery that biopsies of psoriatic lesions express significantly more CRH compared to unaffected skin.33 CRH in unaffected skin stimulates IL-6 and IL-11 proinflammatory cytokines, most likely through increased expression of NFκB in keratinocytes at a local level.34 CRH also appears to increase keratinocyte intracellular adhesion molecule-1 (ICAM-1) expression, promoting immune cell migration and facilitating cell-mediated immune responses.35 It is well documented that CRH stimulates production of pro-opiomelanocortin, which leads to ACTH production and glucocorticoidogenesis in the skin, but this signalling pathway was shown to be defective in psoriasis patients, possibly contributing to inflammation.36 Some have hypothesised that ultraviolet B radiation increases pro-opiomelanocortin, ACTH, and glucocorticoid expression in the skin, resulting in an overall reduction of inflammation within the plaques.37
Another plausible mechanism by which psychological distress induces inflammation is through the dysfunction of cortisol receptors downstream from the HPA axis. There are two intracellular receptor subtypes to which cortisol can bind to exert an anti-inflammatory response: mineralocorticoid receptors (MR, Type I) and glucocorticoid receptors (GR, Type II).38 MR have approximately 10-times more affinity to glucocorticoids and, as such, serve as the primary mediator of negative feedback in the HPA axis at lower glucocorticoid concentrations; however, cortisol levels can often increase 100-fold during times of stress, oversaturating MR and forcing the body to rely on lower-affinity GR to regulate the HPA axis.39 This can ultimately reduce the body’s ability to regulate corticosteroid levels through negative feedback, resulting in decreased sensitivity to the anti-inflammatory effects of cortisol. HPA axis hyperactivity leads to an eventual decline in GR function, which stimulates the expression of NFκB and proinflammatory cytokines. These mediators subsequently contribute to an increasingly inflammatory milieu and fuel rapid keratinocyte proliferation, as seen in cases of worsening psoriasis.40 The potential role of these receptors is supported by clinical data demonstrating that depressed patients are more resistant to the anti-inflammatory effects of dexamethasone, a potent GR agonist, and continue to express proinflammatory cytokines despite administration of this treatment.32 HPA axis hyperactivity thus causes patients to have higher baseline cortisol levels but concomitant desensitisation to its anti-inflammatory effects. In psoriasis, patients with self-identified stress-responsive disease showed blunted cortisol production in response to stress, which is likely to be a consequence of desensitisation.41 In addition, this desensitisation can result in a failed negative feedback loop leading to increased CRH, which can have significant local inflammatory effects on the skin, as previously discussed. These findings corroborate clinical studies that have demonstrated increased cortisol levels in patients with psoriasis and a dose effect positively correlating bedtime cortisol levels with Psoriasis Area Severity Index (PASI) score.42,43 These studies, however, were limited due to small sample sizes and a lack of control groups.
Activation of the sympathetic nervous system in response to psychological stress may contribute to subacute flares of psoriasis.44 Psychological distress, anxiety in particular, has been correlated with sympathetic activation.45 A similar increase in sympathetic tone has been documented in depressed patients, correlating with severity of depression and diminishing when depressive symptoms were successfully treated with selective serotonin reuptake inhibitors (SSRI).46,47 Similarly, psoriatic patients have been found to release more noradrenaline as a response to stress when compared to the general population.48,49 Noradrenaline stimulates alpha adrenergic receptors on antigen-presenting cells and results in decreased expression of CC16 (uteroglobin), a potent anti-inflammatory protein involved in cell-mediated immunity and working in conjunction with the anti-inflammatory cytokine IL-10.50-52 Through this pathway, a subsequent increase in inflammatory IL-6 and TNF-α can be observed, which drives a strong Th1 response.53 Additionally, noradrenaline has stimulatory effects on dendritic cell migration and T cell activation, even when the effect is isolated from those of glucocorticoids alone.54 Noradrenaline also opposes its own inflammatory effects by stimulating beta adrenergic receptors, resulting in a suppression of TNF-α release and increase in IL-10 production.50 It is interesting to question whether interruption of this self-regulatory mechanism may help explain why psoriasis severity can be exacerbated by the use of beta-blockers.55
There are several retrospective case–control studies that correlate psoriasis flares to recent stressful life events;17,18 for example, patients with a larger psychological burden experienced onset of psoriasis at a younger age.56 However, due to the nature of retrospective case–control studies, recall bias is difficult to control and likely overestimates the link between stress and psoriasis. Until recently, a significant controlled, prospective study linking stress to psoriasis had not been performed.57 A small, prospective study following nine women with what was believed to be stress-induced moderate psoriasis showed no relationship between perceived stress levels and timing of psoriasis exacerbations.58 For psoriatic arthritis, however, a 25-year prospective study showed that psoriatic patients with depression were around 37% more likely to develop psoriatic arthritis.59 Given the strong link between psoriatic skin disease and psoriatic arthritis, it is plausible to suspect that psychological distress plays a similar role in both entities.60
In cases when depression leads to worsening of inflammation and psoriasis symptoms, treatment of depression should logically improve outcomes for psoriatic patients. As expected, depressed patients who responded to treatment with the tricyclic antidepressant (TCA) amitriptyline showed a significant reduction in plasma TNF-α, IL-6, and IL-1β.61 An improvement in depressive symptoms correlates significantly with a drop in TNF-α, and a resistance to TCA treatment was associated with higher baseline IL-6.62 This effect is not limited to TCA alone and has also been documented with SSRI, monoamine oxidase A inhibitors, and atypical antidepressants.63,64 Antidepressants not only decrease overall inflammation but also cause a change in the characteristics of that inflammation, reducing IL-12 and consequently inhibiting Th1 cells that are needed for the cell-mediated immune response.65,66 The notion that antidepressant treatment can help psoriatic disease was supported by the results of a double-blind controlled trial in which the addition of a monoamine oxidase A inhibitor resulted in significantly greater reductions in PASI scores after 6 weeks compared to monotherapy with topical corticosteroids alone (p=0.025).67 An open-label study of bupropion, a noradrenaline-dopamine reuptake inhibitor, showed effectiveness as a monotherapy in the treatment of psoriasis, with 8 out of 10 patients reporting a mean PASI score reduction of 50%, which returned to baseline 3 weeks after treatment cessation.68 Of note, case reports have since resulted in warnings about the possible induction of erythrodermic pustular psoriasis with the use of bupropion.69 In a retrospective cohort study of 69,830 Swedish patients with psoriasis, patients exposed to SSRI were 66% less likely to require systemic treatments in the future and more likely to be tapered off systemic treatments during follow-up.70 Considering the high cost of systemic treatments, such as biologics, antidepressants could play an important role in the cost-effective management of depressed psoriasis patients prior to starting more expensive systemic treatments, particularly for patients who see cost as a significant barrier.71
INFLAMMATION CAUSING PSYCHOLOGICAL DISTRESS
While depression may induce worsening of inflammation in psoriasis patients, the opposite is also likely and there is sufficient evidence to suggest that inflammation may be a strong aetiologic factor for psychological distress. Earlier studies showed that a low-grade, cell-mediated immune response causing diffuse oxidative damage could often result in depressive-like ‘sickness behaviours,’ such as psychomotor retardation, anorexia, weight loss, sleep disturbance, and loss of energy.72 Since then, further investigation has revealed multiple other factors that play a role in the induction of these depressive symptoms, including decreased antioxidant levels, increases in oxidative and nitrosative stress, zinc deficiency, and decreased activation of indoleamine 2,3-dioxygenase.73 Mice exposed to lipopolysaccharide and/or IL-1 (as a way to induce inflammatory cytokine release) showed more depressive symptoms, an effect that may have played an important evolutionary role since the display of sickness behaviour would theoretically conserve energy during times of illness or infection.74,75 IL-17A, which has been implicated in the pathophysiology of psoriasis, has been shown to stimulate depressive symptoms in mice;16,76 it is thought this occurs by activation of the NFκB/p38 MAPK inflammatory pathway in brain regions associated with psychological distress, including the hippocampus and prefrontal cortex.16 Furthermore, when mice were treated with antibodies against IL-17A, they were significantly less likely to develop depressive symptoms.76 In humans, however, there has been concern that the IL-17A blocker brodalumab may result in increased suicidal behaviour because four patients completed suicide during Phase III clinical trials.77 Since then, the general consensus is that there does not seem to be a meaningful association between suicidal behaviour and IL-17A blockers.78 In fact, the opposite has also been observed, with a prospective Phase III clinical trial finding that depressive symptoms significantly decreased after 12 weeks of treatment with brodalumab.79 The U.S. Food and Drug Administration (FDA) has since approved the use of brodalumab for psoriasis, although with a black box safety warning for suicidal ideation.
As previously mentioned, mice that were treated with IL-1 antagonists showed a decreased fear response and decreased propensity toward learned helplessness, suggesting that inflammation is important for animals to fully express depressive symptoms.23 These results are not limited to animals and seem to relate to humans also. Among cancer patients, those who were exposed to IFN and/or IL-2 showed significantly more psychological distress and cognitive disturbances due to stimulation of their immune system.80,81 In addition, IFN has long been used to stimulate cell-mediated immunity against the hepatitis C virus82 and in patients with hepatitis C, depression has been detected in up to 80% of those receiving IFN therapy.83 Similarly, depressive symptoms correlated with inflammatory markers like IL-6 and TNF-α in patients vaccinated for Salmonella typhi, even if they showed no physical signs of sickness.84 Mice with an IL-6 gene knockout were much more resistant to psychological distress and less likely to develop depressive symptoms, demonstrating that inflammatory cytokines play a direct role in the development of depression.85 In line with this, postmortem mRNA analyses of the prefrontal cortex of teenage suicide victims revealed an overexpression of IL-1β, IL-6, and TNF-α.86 The reason for these findings may be related to the understanding of how inflammatory cytokines affect important neurotransmitters in the brain.
Systemic inflammation can cause an activation of indoleamine 2,3-dioxygenase, leading to increased breakdown of the serotonin precursor tryptophan into kynurenine, effectively reducing its availability for serotonin production.87 The effect of this functional serotonin decrease is further compounded by the demonstrated ability of kynurenine to serve as a serotonin receptor antagonist and induce de novo depressive symptoms despite the presence of ample serotonin.73 Additionally, kynurenine is subsequently broken down into quinolinic acid, a neurotoxin known to build up in the anterior cingulate gyrus of depressed patients.88,89 Contributing further to this shift, IL-6 increases the breakdown of serotonin in the brain.90 Therefore, inflammation simultaneously decreases serotonin production, increases serotonin breakdown, and inhibits serotonin receptors through a strong synergistic effect.91 This mechanism may explain why inflammation has been associated with significant resistance to several antidepressants.92,93
If inflammation plays a significant role in the severity of depression, treating inflammation would intuitively cause a decrease in depressive symptoms. In a double-blind study, patients taking etanercept, an anti-TNF, showed at least a 50% improvement in the Beck Depression Inventory (BDI) when compared to placebo.94 Improvements in depressive symptoms were not strongly correlated with improvements of objective measures of psoriasis severity, such as PASI. This suggests that improvements in depressive symptoms are a result of inhibition of inflammation and not solely a consequence of decreased disfigurement from disease. This notion is supported by the finding that phototherapy had no significant effect on patients’ depression and anxiety symptoms despite significant improvements in the severity of their psoriasis.95 The interventions that improve psoriasis-associated depression are those that treat the underlying systemic inflammation and not simply the clinical manifestations of the disease.
Further investigation on the matter has shown that the use of anti-inflammatory agents may be viable in depressed patients but treatment selection may be best guided by the identification of subgroups that could respond better to such therapies.96 Clinical trials investigating this seem promising, as one randomised controlled trial testing infliximab for treatment-resistant depression found that the subgroup of patients with particularly high inflammatory markers experienced significant reductions in depressive symptoms after exposure to the TNF-α antagonist.97 Moreover, while severity of psoriasis did not correlate with depression, treatment with ustekinumab, an anti-IL-12 and IL-23 biologic, resulted in a significant decrease in depressive symptoms.98 More recently, guselkumab, an anti-IL-23 monoclonal antibody, was shown to reduce depression and anxiety after 16 weeks in a Phase III randomised double-blind placebo-controlled study.99 These results imply that biologics could be a viable treatment option for psoriasis and the depression that is commonly associated with it.
CONCLUSION
There is undoubtedly a strong correlation between psoriasis and psychological distress. While more research is needed to determine the extent to which psychological distress causes the inflammation seen in psoriasis, or vice versa, one finding remains clear: both depression and psoriasis are inflammatory diseases at the basic, physiologic level (Figure 1). Psoriasis has historically been considered a purely dermatologic condition, often treated with topical steroids for temporary symptomatic relief. However, the paradigm has shifted to treating this disease more like a syndrome, with significant medical and psychiatric comorbidities spanning multiple body systems. The use of biologics and systemic anti-inflammatory drugs has been shown to reduce not only the severity of psoriatic lesions but the risk of developing serious comorbidities, such as myocardial infarction.100 With these medications, dermatologists may now work towards fully treating the psoriasis patient, extending their attention and care further than the skin.
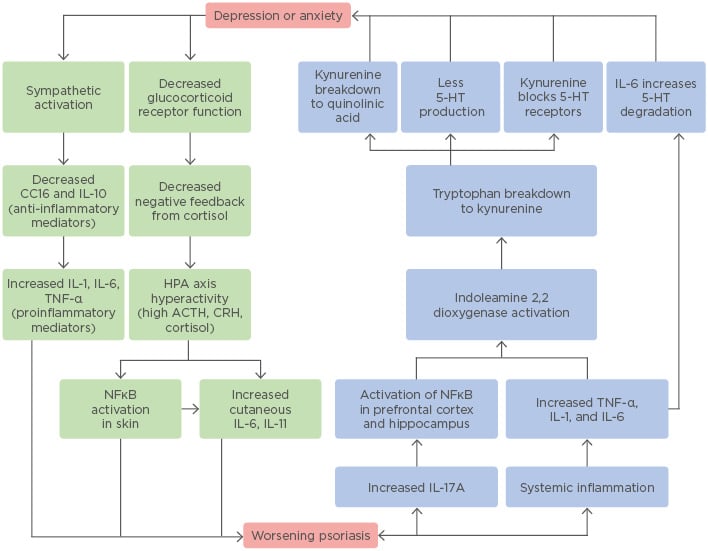
Figure 1: The bidirectional relationship between psoriasis and depression or anxiety.
This flowchart provides an illustrative summary of the biological mechanisms outlined and discussed in this review and demonstrates the complex interactions between these very different clinical entities.
5-HT: 5-hydroxytryptamine (serotonin); ACTH: adrenocorticotropic hormone; CC16: uteroglobin; CRH: corticotropin-releasing hormone; HPA: hypothalamic–pituitary–adrenal.
While treatment of psoriatic skin lesions would be expected to lessen psychological distress, the psychiatric and psychosocial morbidities experienced by patients have not reliably shown to be proportionate to the extent of their cutaneous lesions.101 Failure to screen for and address psychiatric comorbidities in the psoriatic population leads to the persistence of significantly reduced quality of life among these patients. Psychiatric referral is a reasonable step in caring for patients with suspected psychological distress, but dermatologists are truly at the frontline. While many dermatologists are uncomfortable prescribing antidepressants, this may be the only route by which patients can receive this valuable treatment, which will not only improve their quality of life but also improve their skin disease.102 As investigation into the mind–skin connection continues, researchers will hopefully uncover the details required to specifically address the inflammation associated with psoriasis and its psychiatric comorbidities.







