Abstract
Background: Herpes simplex labialis is a common skin condition caused by the herpes simplex virus. The prescription of antivirals for the treatment of herpes labialis is common. The objective of this study was to conduct a systematic review of the available evidence on the treatment of herpes simplex labialis with U.S. Food and Drug Administration (FDA)-approved topical antibiotics.
Methods: The literature search included searches of PubMed, Google Scholar, and Scopus. This review included studies that examined herpes labialis lesions and treatment with topical acyclovir, penciclovir, or docosanol in at least one of the study arms.
Results: Of the 1,485 papers initially identified, 20 papers representing 19 randomised controlled trials and one quasi-randomised trial met the inclusion criteria for the systematic review.
Conclusion: Our systematic review of the clinical studies performed on the three topical antiherpetics, acyclovir, penciclovir, and docosanol, showed that their efficacy compared to placebo is marginal at best (shortening the duration of pain by <24 hours), although the three topical antiherpetic drugs have no serious adverse reactions and are safe to use.
INTRODUCTION
Herpes labialis is primarily caused by herpes simplex virus Type I (HSV-1). Approximately 20–40% of adults are affected at some point in their lives.1 There is currently no cure for herpes labialis outbreaks.2 There is a wide variety of prescription and non-prescription medications used to treat herpes labialis. Topical antivirals, including U.S. Food and Drug Administration (FDA)-approved acyclovir, penciclovir, and docosonal, are often used to treat herpes labialis infections.
Acyclovir is a cyclic guanine nucleoside analogue that lacks the 2’ and 3’ positions normally supplied by ribose.3 Acyclovir inhibits synthesis of viral DNA. This inhibition depends on interactions with thymidine kinase and DNA polymerase.3 Elimination half-life of systematically administered acyclovir is approximately 2.5 hours in adults with normal kidney function.3 Acyclovir is available in intravenous, oral, topical, or ophthalmic (not currently approved in the USA) treatments. Topical acyclovir is prepared as a 5% cream and ointment.2
Penciclovir is an acyclic guanine nucleoside analogue and is similar to acyclovir in potency and activity against HSV. Penciclovir inhibits viral DNA synthesis through competitive inhibition of viral DNA polymerase.3 The half-life of penciclovir is approximately 7–20 hours,3 and is available in a topical form, approved as a 1% cream.2
Docosonal is a long-chain saturated alcohol that inhibits the replication of HSV (lipid-enveloped virus).3 Docosonal is approved as a topical 10% cream for treatment of the orolabial form of HSV only.4 It is the only over-the-counter agent approved by the FDA for the treatment of HSV. As shown in Table 1, all three FDA-approved topical treatments are available in cream form and acyclovir is also available as an ointment. The bases and strengths differ, and the prices range from approximately \$10 up to almost $200.5-11
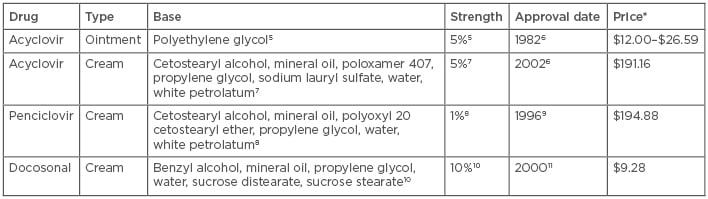
Table 1: Drug characteristics and information.
*Drug prices correct as of 07/06/2018; data obtained from LexiComp Online. Price of preparation is per gram.
The above pricing represents current commercially available products. Please note that products used in the studies may vary from what is currently commercially available.
Several systematic reviews focussing on the effectiveness of antivirals for the prevention of recurrent herpes labialis have been published. However, little has been published on treatment.12 Worrall1 published a systematic review looking at the effects of interventions aimed at preventing recurrent attacks of herpes labialis and found limited evidence that topical antiviral agents reduce healing in herpes labialis recurrent episodes. They also noted that the results from topical antiviral agents were inconsistent and of marginal clinical importance. Since there are a limited number of systematic reviews available that focus on treatment, this systematic review examines the current available evidence of the clinical effectiveness of topical FDA-approved antivirals for the treatment of herpes labialis in adults.
METHODS
Literature Search
The authors conducted this systematic review in accordance with the PRISMA recommendations, which represents a standardised method and format for authors to report systematic reviews.13 PubMed, Google Scholar, and Scopus (which includes content from the Embase database) were searched; the search was limited to the English language, with no time limitation of literature search. The last search was performed in May 2018.
The following search strategy comprising the MeSH and keywords was used: ((“penciclovir” [Supplementary Concept] OR “penciclovir”[Tiab] OR “Danavir”[Tiab]) OR (“Acyclovir”[Mesh] OR “acyclovir”[Tiab]) OR (“docosanol” [Supplementary Concept] OR “Tadenan” [Supplementary Concept] OR “abreva”[Tiab] OR “docosanol”[Tiab])) AND (“Herpes Labialis”[Majr] OR “herpes labialis”[Tiab] OR “cold sore*”[Tiab] OR “fever blister*”[Tiab]). References of all included articles were scanned for additional studies.
Selection
Reviewers included prospective randomised controlled trials (RCT) and quasi-randomised trials with no limitation for sex or country of origin, but excluded trials that examined individuals <13 years old. Studies that examined herpes simplex labialis lesions and included topical acyclovir, penciclovir, or docosanol in at least one of the study arms were included. Studies on comparison among these three topical antiviral agents were also included. Studies on artificially induced lesions, prevention of herpes lesions, and use of herbal therapies, self-concocted drugs, or non-FDA approved drugs were excluded. In vitro studies and studies that required application of the topical medication by a special device were also excluded.
A total of 1,485 unique articles found through the database search were independently reviewed. Studies were selected based on eligibility criteria, data sources, study methods, sample sizes, types of intervention, and authors’ conclusions.
Outcomes
The reviewers looked at the duration of episode and time taken for the lesion to heal, duration of pain, time to loss of crust, and other findings reported for each selected article.
Assessment for Risk of Bias
The reviewers evaluated the studies for risk of bias. Evaluation was based on Cochrane Collaboration’s tool for assessing risk of bias.14
RESULTS
The results of the literature search are shown in Figure 1. Initially, 1,485 potentially relevant articles were identified through the database search. After reviewing the titles and abstracts, 1,419 articles were excluded and 66 full-text articles were eligible for detailed examination. Out of the 66 full-text articles reviewed, 46 articles were excluded based on the criteria cited in Figure 1. Overall, 19 RCT and one quasi-randomised trial met the criteria for systematic review. The characteristics of the 20 studies included in the systematic review are summarised in Table 2 (Click Here to view).15-34
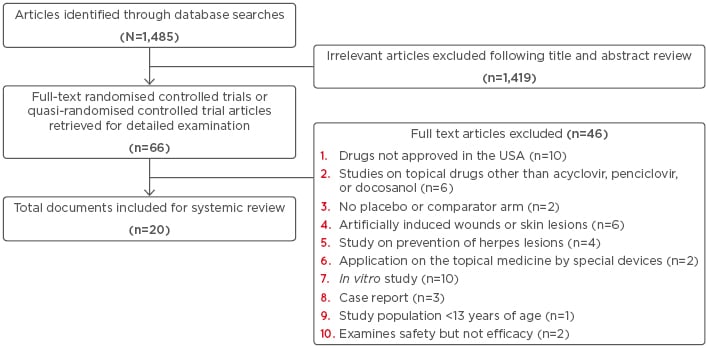
Figure 1: Flowchart representing the literature search carried out during the review.
Risk of Bias
The reviewers evaluated each included study for risk of bias (selection bias, performance bias, detection bias, attrition bias, and reporting bias) based on Cochrane Collaboration’s tool for assessing risk of bias.14 Out of the 20 articles included for systematic analysis, 15 were at low risk of bias, 4 at high risk, and 1 at unclear risk. The 4 studies at high risk of bias were all related to acyclovir (two committed attrition bias, one committed performance bias, and one committed both selection and performance bias).15,20,28,30 The authors considered the study conducted by Habbema et al.33 at unclear risk. Although the authors of this paper mentioned that “…patients were allocated at random on a double-blind basis…”, there was no further description of how the randomised controlled trial was conducted.
Adverse Events
In the 20 studies that were reviewed, all three topical agents (acyclovir, penciclovir, and docosanol) were well tolerated. Most subjects had no reaction or minimal localised reactions that occurred at rates similar to placebo. Localised reactions included inflammation and dry skin. There were no systematic adverse events reported in any of the studies.
DISCUSSION
Acyclovir was evaluated in 14 studies, all of which were RCT. The results of these trials were mixed, with most studies showing no effect or modest improvement with acyclovir treatment. The studies varied in what type of base the acyclovir was prepared in, which may account for some of the variation in results. There were four studies reviewed regarding penciclovir. Two of the studies compared penciclovir to acyclovir and one study showed that penciclovir was superior to acyclovir; however, the other trial showed no difference in effectiveness.30,31 The two other studies regarding penciclovir showed modestly improved healing and pain outcomes when compared to placebo.29,32 Docosanol was compared to placebo in two studies included in this review and had conflicting results; one trial found significantly shorter healing time when compared to placebo, while the other study did not show a significant difference.33,34
All the studies included were prospective in nature. Two of the acyclovir studies included recurrent episodes in their analyses.23,24 Prior to study enrollment, patients had 2–7 recurrences per year in the studies in which this information was specified.18-22,25-34 Most of the studies included treatment with the topical antiviral products 4–8 times per day for an average duration of 4–10 days, and the majority of patients started treatment as soon as possible after symptoms developed, with a few exceptions (see Table 2 for details). None of the studies indicated the patients had associated conditions along with herpes labialis. One of the acyclovir studies was completed in immunocompromised patients;17 however, the other studies were completed in immunocompetent patients.
Chen et al.35 performed a systematic review and meta-analysis to evaluate the effectiveness of nucleoside antiviral drugs for the treatment of herpes labialis. They included 16 publications in their review that included both oral and topical treatments. Oral and topical antivirals shortened the disease course and blocked lesion progression. The only significant difference between oral and topical treatments was a reduction in the healing time of all lesions when using oral medication.
Jensen et al.36 performed a review of oral antivirals for the treatment of recurrent herpes labialis episodes. They reviewed five placebo-controlled and two comparative studies and concluded that treatment with oral antivirals decreased the lesion duration by about 1 day with modest clinical implication.
Rosa et al.37 published a systematic review on 5% acyclovir–1% hydrocortisone cream compared to placebo for herpes labialis treatment. Their meta-analysis showed that early treatment with 5% acyclovir–1% hydrocortisone was beneficial. However, their systematic review was limited to two studies.
This systematic review of 20 trials was limited to FDA-approved topical antivirals for treatment. The reviewers found that topical antiviral therapy has little benefit to treatment. Similarly, Rahimi et al.,12 in their systematic review and meta-analysis reported a lack of benefit from topical antiviral therapy for prevention.
Some limitations of this review were that the systematic review is retrospective in nature, compares only studies that have been previously published by others, and does not prospectively compare the topical treatments. Furthermore, there were only a few studies that met the inclusion criteria for penciclovir and docosanol; most of the studies used acyclovir. Another limitation is that many studies assessing the efficacy of topical antibiotics have heterogeneic study designs, which makes comparison across studies difficult. This review did not investigate the effect of oral FDA-approved antivirals. Worrall’s1 2009 review reported that oral antiviral treatments are more beneficial than topical agents for treatment. However, oral antiviral tablets are available only by prescription in most countries.38
CONCLUSION
It is well known that, unlike herpes zoster lesions (shingles), the majority of immunocompetent patients who develop recurrent herpes labialis lesions have mild local symptoms and the lesions eventually heal without sequelae, even without receiving systemic or topical treatment. This systematic review of the clinical studies evaluating the three topical treatments, acyclovir, penciclovir, and docosanol, also supports the notion that their efficacy compared to placebo is marginal at best (shortening the duration of pain by <24 hours), although the three topical antiherpetic drugs have no serious adverse reactions and are safe to use. It was noted that there is a lack of studies comparing the commercially available treatment options, as most of the studies compared active treatment with one of the three agents to placebo. Although none of the studies looked specifically at cost effectiveness based on the minimal clinical benefit, the self-limiting nature of lesions, and the high cost of medications, the authors would be hesitant to routinely recommend the use of topical antiviral medications for the treatment of herpes labialis.






