BACKGROUND
Nivolumab is a humanised IgG4 monoclonal antibody that binds the programmed cell death protein 1 (PD-1) receptor and blocks interaction with its ligand (PD-L1), preventing the inhibition of T lymphocytes by tumour cells. It is approved for the treatment of various cancers such as melanoma, squamous cell carcinoma of the head and neck, and nonsmall cell lung cancer, among others. The authors report two cases of nivolumab-induced cutaneous toxicity, which has been scarcely reported in the literature.
CASE STUDY
A 66-year-old male with no dermatological history of interest, diagnosed with a Stage IV squamous cell carcinoma of the floor of the mouth, under treatment with nivolumab, presented with a progressive cutaneous eruption on the hands, feet, and scalp (Figures 1A-B). Physical examination revealed erythematous, desquamative, hyperkeratotic lesions on the scalp, fingers, and toes, with intense nail and periungual involvement associated with enthesitis symptoms. Given the suspicion of psoriasiform reactions triggered by nivolumab, topical treatment was initiated, which led to a slight improvement.
A 66-year-old female with a history of cervical carcinoma in situ, ductal carcinoma in situ of the left breast, and invasive lobular carcinoma of the right breast, who was diagnosed with Stage IV lung adenocarcinoma, presented with a 2-month history of a pruritic cutaneous eruption. The patient was under treatment with nivolumab due to disease progression despite having received chemotherapy for a total of 24 cycles. Physical examination showed annular papulosquamous and crusted plaques on the upper torso, limbs, and face (Figures 1C-D). Histopathology showed pseudoepitheliomatous hyperplasia, a lymphoid inflammatory infiltrate in the upper dermis, basal vacuolar change, and abundant apoptotic keratinocytes. The patient tested positive for antinuclear autoantibodies (1/320) and anti-Sjögren’s syndrome-related antigen A (anti-SSA/Ro60). The overall clinical presentation was consistent with a diagnosis of nivolumab-induced subacute cutaneous lupus erythematosus. Nivolumab was discontinued and treatment with topical betametasone dipropionate or gentamicin sulfate, and 1 mg/kg/day oral prednisone was initiated, with resolution. Nivolumab treatment was resumed, but the patient subsequently presented with pneumonitis, leading to discontinuation of the drug indefinitely.
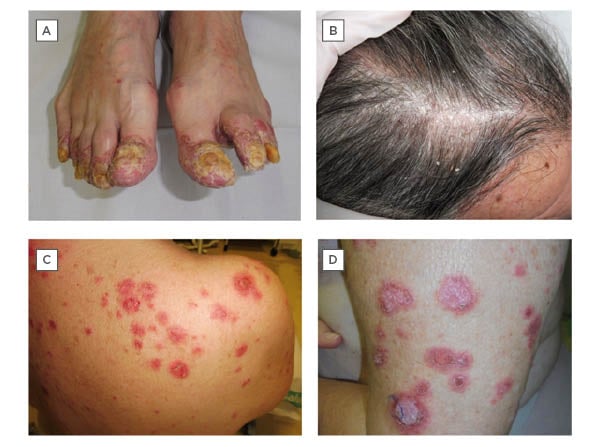
Figure 1: A-B) Erythematous, desquamative, hyperkeratotic lesions on scalp and toes, with intense nail and periungual involvement in a patient with a psoriasiform reaction triggered by nivolumab. C-D) Annular papulosquamous and crusted plaques on the upper trunk and limbs in a patient with a nivolumab-induced subacute cutaneous lupus erythematosus.
The most common patterns of cutaneous toxicity caused by PD-1 inhibitors are lichenoid dermatoses, pruritus, and vitiligo.1,2 Other dermatoses such as bullous pemphigoid, or the psoriasiform reactions3 and subacute cutaneous lupus erythematosus4,5 that were presented, are much less frequent. In the case of psoriasiform reactions, the differential diagnosis with paraneoplastic acrokeratosis can be complicated, as was the case for this patient. It is therefore important for dermatologists to know the cutaneous adverse effects of these new generation of oncological therapies such as nivolumab. A multidisciplinary approach to the cutaneous toxicities of these drugs facilitates early diagnosis and effective management, which allows patients to remain on these survival-prolonging treatments. It is expected that, given its increasing use, other adverse effects will be described in the future.







