Author: Alberto Catapano1,2
1. Center of Epidemiology and Preventive Pharmacology University of Milan, Italy
2. Laboratory of Lipoproteins, Immunity andAtherosclerosis, University of Milan, Italy
Speakers: Chiara Giannerelli,1,2 Eicke Latz,3 Wolfgang Koenig,4 Amit Khera,5
1. New York University Langone Health, New York, USA
2. New York University Grossman School of Medicine, New York, USA
3. Institute of Innate Immunity, University of Bonn, Germany
4. German Heart Center Munich, Technical University of Munich, Germany
5. Massachusetts General Hospital, Boston, USA
Disclosure: Catapano has received research grant(s) or support from Akcea, Amarin, Amgen, Menarini, Mylan, Sanofi, and Sanofi/Regeneron; and has served as a consultant for Akcea, Amgen, Amarin, Daiichi-Sankyo, Eli Lilly, Esperion, Kowa, Ionis Pharmaceuticals, Menarini, MSD, Mylan, Novartis, Recordati, Regeneron, and Sanofi. Giannerelli has a patent related to part of her presentation PCT/US22/17777. Latz is a co-founder, consultant, or member of the board for DiosCURE, IFM Therapeutics, Odyssey Therapeutics, and Stealth Biotech. Koenig has received honoraria or expenses from Amgen, AstraZeneca, Bristol Myers Squibb, Novartis, Roche, and Sanofi; has consulted or served on an advisory board for Amgen, AstraZeneca, Corvidia Therapeutics, Daiichi Sankyo, DalCor, Esperion Therapeutics, Genentech, Kowa, Novartis, Novo Nordisk, OMEICOS Therapeutics, Pfizer, and The Medicines Company; and has received research funding from Abbott Diagnostics, Dr. Beckmann Pharma, Roche Diagnostics, and Singulex. Khera has consulted or served on an advisory board for Amgen, Color Health, Foresite Labs, Novartis, and Silence Therapeutics; has received research funding from IBM Research and the National Institutes of Health (NIH); and holds stock options and equity in, and is an employee of, Verve Therapeutics.
Acknowledgments: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: This independent medical education activity is provided by EMJ, and was supported by independent funding from Novartis Pharma AG, who have had no input into the content. This educational activity is intended for an audience of non-US healthcare professionals. These materials may include data/information on investigational uses of compounds/drugs that have not yet been approved by regulatory authorities.
Citation: EMJ Cardiol. 2022;10[Suppl 3]:02-10. DOI/10.33590/emjcardiol/22C3203. https://doi.org/10.33590/emjcardiol/22C3203.
Meeting Summary
This plenary session began with a focus on cutting edge research into the role of the immune system in atherosclerotic cardiovascular disease (ASCVD). Firstly, Chiara Giannerelli, New York University (NYU) Langone Health and NYU Grossman School of Medicine, New York, USA, emphasised the value of deep phenotyping of atherosclerotic disease to understand the immune mechanisms involved in cardiovascular (CV) risk. She presented data demonstrating an enrichment of phenotypically distinct cluster of differentiation (CD) 4+ and CD8+ T cells in advanced atherosclerotic plaque compared with paired blood and explained how this data can be used to identify drugs with the potential to be repurposed to reduce CV risk.
Secondly, Eicke Latz, Institute of Innate Immunity, University of Bonn, Germany, presented data from his research team that begins to explain how the Western diet might lead to chronic inflammation and atherogenesis, implicating cholesterol crystals and short-chain sphingomyelins in the reprogramming of granulocyte-monocyte precursor cells (GMP) in this process. The arguments for greater use of imaging and molecular biomarkers in clinical practice were presented by Wolfgang Koenig, German Heart Center Munich, Technical University of Munich, Germany, who speculated that the assessment of CV risk in the future is likely to harness big data and machine learning to achieve accurate risk assessment in individual patients.
Finally, Amit Khera, Massachusetts General Hospital, Boston, USA, covered the role of genetic factors in the prediction of CV disease (CVD), explaining that a combination of traditional risk factors and polygenic scoring techniques provides the most accurate estimation of CVD risk.
Deep Phenotyping of Atherosclerotic Disease
Chiara Giannerelli
Giannerelli emphasised that understanding the cellular and molecular immune mechanisms involved in atherosclerotic plaque erosion and rupture are crucial to identify novel treatment approaches. However, she explained that the precise role of the immune system at different stages of atherosclerosis remains unclear, partly because plaque erosion and rupture are phenomena exclusive to humans and are therefore difficult to study in animal models.1
Clinical trials have shown that targeting inflammation can be very effective in reducing CV risk in patients with atherosclerosis.2-4 However, they have also illuminated that effective treatment requires an understanding of the immune mechanisms relevant at different stages of atherosclerosis.5-7 For example, the COPS trial5 and the COVERT-MI study6 showed that colchicine was not effective in reducing CV risk in the acute phase of the disease, and the CIRT study7 showed that low-dose methotrexate after myocardial infarction (MI) was not sufficient to reduce inflammatory markers.
Many factors influence plaque characteristics, including genetics, epigenetics, somatic mutations, diet, and lifestyle, and this results in complex plaque pathology, making it challenging for pathological techniques to fully understand the differences between a plaque associated with a high risk of major adverse cardiovascular events (MACE) and one with a low risk.8 Giannerelli stressed that deep phenotyping of atherosclerotic disease is essential to fully understand the immune mechanisms involved in CV risk in patients with atherosclerosis, and potentially to guide new treatment approaches.
Deep Phenotyping of Atherosclerosis Using Single-Cell Technologies
In Giannarelli’s laboratory, immune phenotyping using cytometry by time of flight showed that CD4+ and CD8+ T cells were enriched in advanced atherosclerotic plaque compared with paired blood (N=23).9 These cells were phenotypically distinct from those in paired blood, expressing higher levels of CD69, showing that they were tissue-resident, and CCR5, showing that they were activated. They also expressed higher levels of programmed cell death protein 1, a target of immune-checkpoint inhibitors used to treat cancer. The CD4+ T cell population in plaque was particularly enriched in patients who had experienced a stroke versus patients who had not experienced a stroke.9
Single-Cell-Driven Drug Repurposing to Target Atherosclerosis
To identify drugs that could be repurposed to treat atherosclerosis, peripheral blood mononuclear cells from patients with CVD were stimulated ex vivo with plasma from the same patients or with plasma from healthy donors (Figure 1).10 The transcriptional inflammatory gene signature of cells stimulated by plasma from patients with CVD was then used to identify potential drugs from the National Institutes of Health (NIH) Library of Integrated Network-Based Cellular Signatures (LINCS) database that could be repurposed for atherosclerosis. Eight candidate drugs were screened for their ability to reverse the molecular signature associated with immune cells in CVD and one of these, saracatinib, was selected for evaluation in animal models.
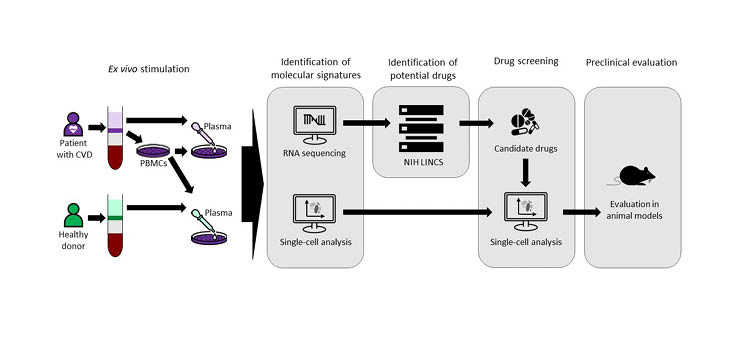
Figure 1: Single-cell-driven drug repositioning to treat atherosclerosis.10
CVD: cardiovascular disease; LINCS: Library of Integrated Network-Based Cellular Signatures; NIH: National Institutes of Health; PBMC: peripheral blood mononuclear cell.
In a mouse model of atherosclerosis, infiltration of macrophages into atherosclerotic plaque was significantly reduced in mice treated with saracatinib compared with those treated with atorvastatin and control mice. 10 In a larger animal model, rabbits were given a high-cholesterol Western diet and aortic endothelial denudation for 16 weeks to induce the formation of complex lesions (Giannarelli, unpublished data). Uptake of 18F-fluorodeoxyglucose into established plaques was significantly reduced after 16 weeks and 28 weeks of treatment with saracatinib plus atorvastatin compared with untreated animals, indicating a reduction in inflammation. In addition, immunohistochemistry showed that combination treatment significantly reduced lipid accumulation in established plaques compared with no treatment.
Giannarelli concluded by saying that new targets for treating atherosclerosis are needed, and that deep phenotyping is one approach made possible by recent technological advancements, which offers the potential to advance precision medicine.
Vascular Response in Atherosclerosis: From Epigenetics to Bone Marrow
Eicke Latz
Latz’s research group focuses on defining the relationships between the triggers for inflammation and how they result in chronic disease. He explained that transcriptional regulation of inflammation involves the production of inflammatory cytokines, some of which remain in the cytosol in an inactive form until they are activated by caspase-1, which itself requires activation by inflammasomes.11
Inflammasomes are intracellular multimeric protein complexes that act as sensors, responding to the presence of unique microbial components by activating caspase-1.11 The nucleotide-binding oligomerisation domain, leucine-rich repeat-containing protein 3 (NLRP3) inflammasome is critical for host immune defences, yet it has also been linked to the pathogenesis of several chronic inflammatory disorders.11 Latz explained that before the NLRP3 inflammasome can be activated, a priming step is needed to induce the formation of the protein complex.12
Inflammation and the Western Diet
Chronic inflammation in gout is known to be associated with activation of the NLRP3 inflammasome by uric acid crystals,13 and this led Latz and his team to investigate whether cholesterol crystals might have a similar effect. They used laser reflection microscopy to show that cholesterol crystals are readily taken up by macrophages, even in early atherosclerotic lesions, and that they activate the NLRP3 inflammasome resulting in IL-1β secretion (Figure 2).12
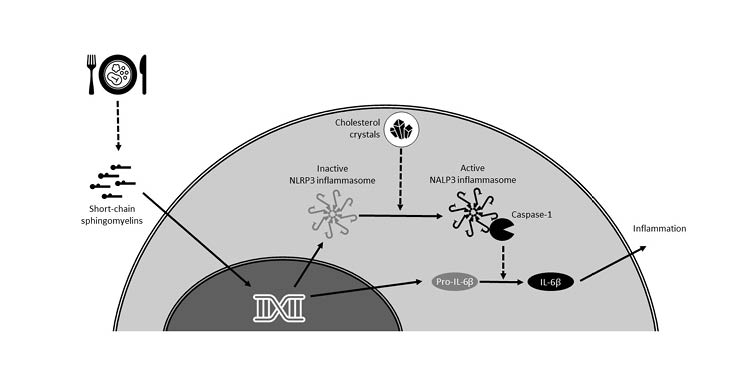
Figure 2: Putative role of a Western diet on inflammation in atherosclerosis.12,14
NLRP3: nucleotide-binding oligomerisation domain, leucine-rich repeat-containing protein 3.
Latz’s team then investigated whether a Western diet associated with high cholesterol levels could trigger an innate immune memory response, which could lead to chronic inflammation. They found that in a mouse model, a 4-week Western diet was associated with a significant increase in the release of both IL-1β and IL-6 from bone marrow cells primed with lipopolysaccharide (LPS). Importantly, this altered response was still apparent 4 weeks after the mice returned to a chow diet, indicating that the Western diet had induced an innate immune memory response.14
A Western diet was also associated with higher numbers of circulating monocytes and granulocytes, as well as GMPs, compared with a chow diet. Chromatin accessibility in GMPs was also different in mice on a Western diet compared with a chow diet, including a more open configuration in enhancer regions Tlr4 and Tet2, and around genes associated with leukocyte differentiation, T cell activation, IL-6 production, and the JAK/signal transducer and activator of transcription proteins pathway. These chromatin changes were still apparent in Western diet-fed mice after they were returned to a chow diet for 4 weeks, supporting the conclusion that, in this mouse model, a Western diet induced an innate immune memory response.14
In this mouse model, NLRP3 knockout abrogated both the initial cytokine response and the GMP activation and reprogramming response to a Western diet, indicating that these responses are NLRP3 dependent.14
To investigate whether these findings also applied to humans, Latz’s team isolated primary monocytes from 120 healthy volunteers. Monocytes were primed with oxidised low-density lipoprotein (LDL), a proinflammatory molecule associated with a Western diet, allowed to rest for 6 days, and then primed with LPS.14 Oxidised LDL priming was found to result in an increased responsiveness to LPS stimulation compared with controls, and single nucleotide polymorphisms were detected in the putative regulatory region of the IL-1 receptor antagonist gene. These findings suggest that IL-1 is likely to be important in driving the NLRP3 inflammasome-associated systemic inflammation induced by a Western diet.
Primers for Inflammation in Atherosclerosis
Using a machine learning discovery approach that exploited the apparent phenotypic variability in models of inflammation, over 1,000 characteristics of 504 mice were analysed to identify characteristics that correlated with atherosclerotic plaque size. This approach illuminated several known risk factors for murine atherogenesis, such as female gender, low high-density lipoprotein, and high IL-1β levels. It also identified several novel metabolic factors associated with atherogenesis, including sphingolipid metabolism. Short-chain sphingomyelin (S14) was highly correlated with atherosclerotic plaque size and produced significant inflammatory cytokine responses in macrophages, compared with longer-chain sphingomyelins. S14 was also shown to induce transcriptional responses and metabolic reprogramming in murine macrophages through a Tlr4-dependent mechanism.
In human macrophages, S14 induced Tlr4-dependent IL-6 release, and a significant reduction in S14 sphingomyelin levels was observed over 12 weeks of a very-low-calorie diet in subjects with Type 2 diabetes and overweight/obesity.
In summary, Latz emphasised that the inflammasome can be activated by cholesterol crystals, potentially with a priming step induced by short-chain sphingomyelins, and this leads to complex reprogramming of GMP cells (Figure 2). Latz proposed that since S14 production can be induced by exposing cells to glucose and insulin to produce fat cells, the Western diet may lead to increased levels of S14, resulting in chronic inflammation and atherogenesis.
Imaging and Molecular Biomarkers: Targeting the Right Patient for the Right Treatment
Wolfgang Koenig
Approximately one-third of CVD risk cannot be explained by traditional CV risk factors such as high blood pressure, high cholesterol, smoking, alcohol intake, and lack of exercise,15 and Koenig explained that greater use of biomarkers may help to refine risk stratification in clinical practice.
The Argument for Greater Use of Imaging Biomarkers
The PESA study first indicated that it may be beneficial to assess individuals for subclinical atherosclerosis.16,17 In this study, 50% of subjects (n=1,779) with no traditional CV risk factors were shown to have subclinical atherosclerosis upon imaging, defined by the presence of atherosclerotic plaques and/or heavy calcification.
The assessment of plaque burden using ultrasound imaging is supported by a population cohort study (n=2,965; mean follow-up: 7.2 years) in which maximum intima-media thickness of the internal carotid artery of >1.5 mm (indicating the presence of plaque) significantly improved upon risk classification for new-onset CVD using the Framingham risk score (net reclassification index: 7.3%; p=0.01).18 Assessment of coronary artery calcification (CAC) provided predictive information for incident coronary heart disease beyond that of traditional risk factors in MESA (n=6,722; median follow-up: 3.8 years).19 In the BioImage Study, both plaque burden and CAC imaging biomarkers improved upon traditional risk factors by two- to three-fold for the prediction of 3-year risk of MACE in an at-risk population (n=5,808).20
Current guidelines for the primary prevention of CVD in clinical practice recommend that individuals at intermediate-risk of CVD should be further assessed with CAC scoring, with the use of plaque burden assessment reserved for situations where CAC is unavailable or not feasible.21 However, Koenig feels that recommendations for the use of imaging biomarkers will soon be expanded.
The Argument for Greater Use of Molecular Biomarkers
Koenig explained that three types of biomarkers have garnered the most interest in the cardiovascular field in recent years. These include markers of myocardial stress and remodelling such as N-terminal pro-B-type natriuretic peptide (NT-pro-BNP); markers of myocardial injury such as troponin; and markers of inflammation such as C-reactive protein (CRP).22
NT-pro-BNP, troponin, and CRP have been shown to significantly improve upon risk prediction using a conventional risk factor model,23 and troponin has also been shown to be associated with an increased risk of CV disease and CV mortality.23,24 However, Koenig pointed out that although these improvements in risk prediction were statistically significant, they were marginal, making it difficult to argue clinical relevance. More recently, a meta-analysis of 28 studies found that subjects with troponin levels in the top third of the distribution had an increased risk of a cardiac event compared with those that had troponin levels in the bottom third (relative risk: 1.43; mean follow-up: 8.2 years).25
Troponin and NT-pro-BNP have also been shown to be important for the prediction of CV mortality in patients with stable coronary heart disease, and the prediction of ASCVD in patients with prior heart attack.26,27
An analysis of long-term data from the WOSCOPS study of men at high-risk of CVD (n=3,318) illuminates how troponin measurements can help clinicians to tailor their therapeutic approach.28 In this study, baseline troponin levels in the upper quartile of the distribution were associated with an increased risk of CV death over 15 years of follow-up (p<0.001). The use of pravastatin 40 mg once daily was associated with a 13% greater reduction in troponin levels over 1 year compared with placebo, and this correlated with improved 5-year outcomes beyond the lowering of LDL cholesterol.
The combined use of vascular inflammatory biomarkers, including CRP, IL-6, TNF, and imaging biomarkers has been shown to enhance the risk discrimination (c-index) for MACE by 3.1 compared with traditional risk assessment.29 Indeed, clinical trials have shown that the use of anti-inflammatory drugs in patients with a previous MI reduced the risk of CV events compared with placebo.2,3
The combination of imaging and molecular biomarkers was recently assessed for the ability to predict ASCVD (n=4512); average follow up of 13.2 years.30 Both CAC score and lipoprotein(a) levels were independently associated with ASCVD risk in asymptomatic subjects, and subjects with both a CAC score ≥100 and lipoprotein(a) levels in the upper quintile had an approximately five-fold increased risk of ASCVD (adjusted hazard ratio: 4.71).
Koenig introduced the European CoroPrevention study, which is due to start recruiting in October 2022.31 This study will stratify subjects with coronary heart disease using a combined biomarker score (including Coronary Event Risk Test [CERT2], based on circulating ceramide and phosphatidylcholine levels; troponin I; NT-pro-BNP; and cystatin C). Subjects considered to be at high-risk of CVD will be randomised to either personalised prevention or usual care, and Koenig hopes that this study will illuminate the potential benefits of biomarkers to inform risk-based therapeutic management of patients.
In the future, Koenig believes that biomarkers for CVD will increasingly be assessed through multimarker protein panels, which can be utilised to predict clinical risk using advanced computational modelling techniques. Initial studies have shown that the use of a proteomics model may be superior to a clinical model when predicting CV risk in both primary and secondary prevention, particularly in the short term (<3 years).32,33
Koenig concluded by saying that in coming years, CV risk is likely to be based on standard risk scores, with the integration of other imaging and/or circulating biomarkers, and will increasingly harness big data and machine learning, with a view to achieving accurate individualised risk assessment and personalised therapy.34
Implementing Genetic Scores in Clinical Practice
Amit Khera
The association of a family history of MI was first linked to an increased risk of CVD in 1951.35 However, it is only in the past few years that scientists have had the tools and data to begin to understand the genetic basis behind this association.
Khera explained that conceptionally, an individual’s DNA might predispose them to MI through a single mutation that disrupts a specific pathway (the monogenic model), or the combined effects of multiple mutations in different pathways (the polygenic model).
To harness present-day cutting-edge technologies, a genome interpretation study of MI was designed based on the original 1951 publication.36 The genomes of subjects with a first MI at age ≤55 years (n=2,081) were compared with healthy controls from a multiethnic population (n=3,761). The monogenic familial hypercholesterolaemia mutation, which prevents clearance of LDL cholesterol from the circulation, was identified in 1.7% of subjects with MI, representing a 3.8-fold increased risk of early-onset MI.36 However, this means that 98.3% of the subjects with early-onset MI could not be identified using this monogenic approach.
To investigate whether a polygenic risk model might be able to identify some of these subjects, a genome-wide polygenic score for coronary artery disease (GPSCAD) was applied to the study. GPSCAD was derived from a large genome-wide association study that included 6.6 million common variants, using computational algorithms.37 The polygenic score was tested on a sample of 300,000 subjects, showing a striking trend of increasing prevalence of CAD in subjects with higher scores. Subjects with a high polygenic score (a score in the top 5% of the population distribution) were shown to have a >3-fold risk of CAD compared with the rest of the population. When GPSCAD wasapplied to the genome interpretation study of MI, 17% of subjects were found to have a high polygenic score, representing a 3.7-fold increased risk of early-onset MI.36
GPSCAD score did not correlate with 10-year risk defined by the Pooled Cohort Equations (PCE) clinical risk estimator for ASCVD, which relies heavily on age and sex.38 Khera explained that this is not surprising when it is considered that 20–30% of the variants included in GPSCAD are associated with pathways that are known to be important in CVD but do not measure routinely, and for another 50% of the variants, it is unclear how they modify biochemical pathways to effect MI risk.
However, when the predictive value of the PCE risk category is overlaid with the GPSCAD score, there is a striking gradient according to the GPSCAD score within each PCE risk category.38 For example, subjects in a UK Biobank population categorised by PCE as ‘intermediate’ risk (7.5–20.0%) of ASCVD could be subdivided into those with a GPSCAD score in the highest quintile, with a risk of 2.8%; those with a GPSCAD score in the intermediate quintiles, with a risk of 4.7%; and those in the lowest quintile, with a risk of 8%. Similar results were seen in a cohort from the Malmo Diet and Cancer Study.38
However, Khera stressed that an individual’s genetics do not predetermine whether they will develop CVD. This is supported by studies that showed that both a healthy lifestyle and the use of lipid-lowering medication could reduce the risk of coronary events in those patients with a high GPSCAD score by approximately 50%.39,40
To investigate whether a higher polygenic risk score might increase the penetrance of a monogenic driver of CVD, Fahed et al.41 analysed the genetics of a case control dataset of 12,852 subjects with prior MI. While the presence of a monogenic mutation (familial hypercholesterolaemia) conferred a three-fold risk of MI across the entire cohort (odds ratio [OR]: 3.2), this risk was much lower in subjects with a low GPSCAD score (OR: 1.3) and considerably higher in those with a high GPSCAD score (OR: 12.6). Applying this combined monogenic/polygenic risk assessment approach to 48,812 UK Biobank subjects confirmed that the polygenic score of an individual modifies the penetrance of a monogenic familial hypercholesterolaemia mutation.
Khera summarised by describing polygenic scoring as a quantitative metric of inherited risk that can be identified at birth, providing many years to work towards offsetting this risk. He suggested that the genetic risk assessment of almost any disease likely needs a combined monogenic and polygenic approach to fully recognise the individual risk of developing the disease in each person.
In heart disease, polygenic scoring can exceed the predictive capability of traditional risk factors, yet Khera stressed that many individuals with a high polygenic score are not currently recognised in clinical practice as being at high risk of CVD.








