Chairpeople: James Januzzi,1 Evangelos Giannitsis,2 Alexandre Mebazaa,3 Martin R. Cowie4
Speakers: James Januzzi, Mårten Rosenqvist,5 Cynthia Papendick,6 Richard Troughton,7 Bonnie Ky,8 Lars Wallentin,9 Raphael Twerenbold,10 Agneta Siegbahn,9 Rick Pleijhuis,11 Philip J. (PJ) Devereaux,12 Carolyn Lam,13 Nasrien Ibrahim1
1. Massachusetts General Hospital, Boston, Massachusetts, USA
2. Heidelberg University Hospital, Heidelberg, Germany
3. Hôpital Saint-Louis & Lariboisière, Paris, France
4. Imperial College London, London, UK
5. Karolinska Institute, Stockholm, Sweden
6. Royal Adelaide Hospital, Adelaide, Australia
7. Christchurch Heart Institute, Christchurch, New Zealand
8. Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania, USA
9. Uppsala University Hospital, Uppsala, Sweden
10. University Hospital Basel, Basel, Switzerland
11. University Medical Center, Groningen, the Netherlands
12. McMaster University, Hamilton, Canada
13. National Heart Centre Singapore, Singapore, Singapore
Disclosure: Dr Januzzi has received grant support, consulting income, and/or served on clinical endpoint committees/data safety monitoring boards for Novartis, Applied Therapeutics, Innolife, Abbott, Janssen, Quidel, Roche Diagnostics, AbbVie, Amgen, CVRx, MyoKardia, and Takeda. Prof Giannitsis has received honoraria for lectures from Daiichi Sankyo, AstraZeneca, Roche Diagnostics, Boehringer Ingelheim, Bayer Vital, and BRAHMS GmbH; receives research funding from Daiichi Sankyo and Roche Diagnostics; and consults Roche Diagnostics, AstraZeneca, Bayer Vital, Idorsia, Radiometer, BRAHMS GmbH, Hoffmann-La Roche, and Boehringer Ingelheim. Dr Mebazaa has received personal fees from Orion, Servier, Otsuka, Philips, Sanofi, Adrenomed, Epygon, and Fire 1; and has received grants and personal fees from 4TEEN4, Abbott, Roche, and Sphyngotec. Prof Cowie has provided consultancy advice to and received speaker fees from Roche Diagnostics, AstraZeneca, Boehringer Ingelheim, Bayer, Servier, Medtronic, Boston Scientific, and Abbott. Prof Rosenqvist has received consultancy fees, received research grants, and/or given lectures sponsored by Abbott, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, MSD, Pfizer, Roche, and Zenicor. Dr Papendick has received research support from Roche Diagnostics, the Australian Government (National Health and Medical Research Council [NHMRC]), and from South Australian Health, plus financial compensation from Roche Diagnostics. Prof Troughton has received grants from Heart Foundation of New Zealand, Health Research Council of New Zealand, Roche Diagnostics, Merck, Sanofi, and Pfizer; and has received honoraria from Merck and Roche Diagnostics. Dr Ky has received personal fees for honoraria from Roche, the American College of Cardiology (ACC), and Uptodate; consultancy fees from Cytokinetics; and grants from the National Institutes of Health (NIH). Prof Wallentin has received grants from Roche Diagnostics, AstraZeneca, Bristol Myers Squibb/Pfizer, GlaxoSmithKline, Merck & Co, and Abbott; personal fees from Boehringer Ingelheim; and has two patents licensed to Roche Diagnostics. Dr Twerenbold has received research support, travel support, and consultancy fees from several diagnostic and pharmaceutical companies. Dr Siegbahn has received institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Roche Diagnostics, and consulting fees from Olink Proteomics. Dr Pleijhuis has received speaker fees from Roche Diagnostics and is a cofounder and shareholder of Evidencio BV. Prof Devereaux has received grants from Abbott Diagnostics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Covidien, Octapharma, Philips Healthcare, Roche Diagnostics, and Stryker; has participated in advisory board meetings for GlaxoSmithKline; and has participated in expert panel meetings for AstraZeneca, Boehringer Ingelheim, and Roche. Prof Lam has received research support and/or served as a consultant or on the advisory board/steering committee/executive committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma, eko.ai, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck & Co, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group, Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma, and WebMD Global LLC; and is cofounder and nonexecutive director of eko.ai. Dr Ibrahim has received honoraria from Novartis and Roche Diagnostics.
Acknowledgements: Writing assistance was provided by Helen Boreham, HB Medical (UK) Ltd, Yorkshire, UK.
Support: The publication of this article was funded by Roche. The views and opinions expressed are those of the presenters. The content was reviewed by Roche for medical accuracy.
Citation: EMJ Cardiol. 2021;9[Suppl 2]:2-12.
Meeting Summary
proCardio is a unique scientific event that focusses on the field of biomarkers in cardiovascular (CV) medicine. Held in 2020 as an interactive online webinar, proCardio featured a world-class faculty of leading international experts who communicated the latest evidence and clinical trial data from the cardiac biomarker arena, with the aim of shaping future clinical pathways and addressing existing unmet medical needs. The virtual proCardio programme spanned 3 days of webinar presentations and featured discussions centred on three key topics: atrial fibrillation (AF), coronary artery disease (CAD), and heart failure (HF).Learnings from STROKESTOP II
Professor Mårten Rosenqvist
Oral anticoagulation is currently underused in AF management, with one-third of cases remaining silent and asymptomatic. The STROKESTOP studies have been devised to assess whether screening for AF in key risk groups, combined with anticoagulation, may decrease the incidence of stroke and other complications. In STROKESTOP II, 28,712 Swedish patients aged 75–76 years old were randomised to either ECG screening or standard of care.1 Testing was also carried out for levels of N-terminal pro B-type natriuretic peptide (NT-proBNP), a key biomarker linked to AF and stroke risk that may be capable of detecting clinically silent AF. Of the 6,868 individuals who attended screening, 8.1% had known AF and new AF was detected in a further 2.6%. In total, 60% of patients had elevated NT-proBNP levels ≥125 ng/L and went on to receive prolonged ECG screening. In this high-risk group, 4.4% were ultimately diagnosed with AF, raising the prevalence of AF in the whole group by around 30% (from 8.1% to 10.5%). A further 93 participants (1.4%) were found to have extremely high NT-proBNP biomarker levels ≥900 ng/L and underwent additional cardiac workup. On referral, 14% of this group were diagnosed with a previously unknown serious cardiac disease, including aortic valve disease and amyloidosis.
In other key findings from STROKESTOP II, unknown hypertension was found to be as common as silent AF in the 75–76-year-old group and pulse palpation was shown to lack accuracy as a clinical tool for AF detection. Overall, these results indicated that NT-proBNP is a sensitive marker for undiagnosed AF, with high levels also acting as a robust risk marker of underlying cardiovascular disease (CVD), Prof Rosenqvist concluded.
Where Do We Stand with the ESC 0/1-Hour Algorithm?
Doctor Cynthia Papendick
Troponin assays have been the biomarker of choice for risk stratification in acute coronary syndrome for the past two decades; however, clinical uptake of newer, high sensitivity troponin (hs-cTn) assays has been hindered by a lack of practical evidence for their application. Dr Papendick reviewed three key studies, two pre- and post-implementation trials (RAPID-CPU and BABA project) and one randomised controlled trial (RAPID-TnT), that provide validation for the safety and efficiency of the European Society of Cardiology (ESC) 0/1-hour algorithm using real-world data.1-4 All studies included undifferentiated patients presenting with potential acute coronary syndrome, excluding those with ST-elevation myocardial infarction or on end-stage dialysis, and used 30-day major adverse cardiac events (MACE) or all-cause mortality as the primary outcome. Results showed a similar percentage of patients triaged across all three studies, with all trials also demonstrating a significant (p<0.001) increase in emergency department (ED) discharge rates and a decreased length of ED stay. Critically, a very low MACE rate (<1%) was observed across all three studies. The impact on downstream diagnostic resource utilisation for grey-zone patients was minimal; however, further work is still needed to delineate a more targeted approach to this ‘observe group’. Dr Papendick explained that grey-zone patients constitute a very heterogenous group but are definitively not low-risk, as illustrated by the 2.3% death and myocardial infarction event rate in the RAPID-TnT trial. Breaking this down by troponin levels revealed that even grey-zone patients with modest rises (values in the range 12–22 ng/L) experienced events. Collectively, these real-world data support the safety, feasibility, and ED flow improvements of the 0/1-hour algorithm. Updated 2020 ESC guidelines reflect this by recommending the 0/1-hour approach first-line to rule out non-ST-elevation myocardial infarction.5
IMPERATIVE-HF: Biomarkers in Heart Failure Post-Discharge Management
Doctor Richard Troughton
Hospitalisation for acute HF is currently complicated by very high rates of readmission and mortality.6 Treatment post-discharge is also suboptimal, with European data illustrating that only one-third of patients receive >50% of optimal doses of guideline-recommended medications.7,8 Against this challenging backdrop, NT-proBNP offers a powerful prognostic biomarker that can aid in HF management. Levels of NT-proBNP at discharge are proven to be a strong predictor of early mortality or rehospitalisation at 180 days, as demonstrated in a meta-analysis of over 1,300 patients with acute HF.9 Changes in NT-proBNP also reflect response to therapy and long-term risk, falling with effective treatment.10 Dr Troughton explained that the IMPERATIVE-HF study was designed to address these existing unmet needs of readmission, mortality, and suboptimal treatment after HF hospitalisation by leveraging the power of NT-proBNP. The multicentre, parallel-group, randomised, open-label, blinded endpoint study enrolled 450 patients with a primary admission diagnosis of acute HF.11 Prior to discharge, patients were randomised to either usual care (UC) or natriuretic peptide (NP)-guided care in which medication was uptitrated to achieve target NT-proBNP levels <1,000 pg/mL. Overall, 21% of the UC cohort experienced the primary endpoint (time to death or HF readmission at 180 days) compared with only 16% in the NP-guided group. Rates of HF readmission and all-cause mortality were also lower in the NP group. Despite the 30% reduction in primary endpoint risk (hazard ratio [HR]: 0.7; 95% confidence interval [CI]: 0.45–1.1) for the NP-guided group, this difference did not reach statistical significance (p=0.1), largely because of the lower-than-expected event rate in the UC arm. In terms of secondary outcomes, the NP group had greater use of guideline-recommended medications at 6 and 12 weeks, including more patients on triple therapy, and achieved higher total doses. Overall, IMPERATIVE-HF showed that a strategy of NT-proBNP guided treatment after discharge was associated with more optimal early treatment and improved clinical outcomes.
The Value of Cardiac Biomarkers in Cardio-Oncology
Doctor Bonnie Ky
Dr Ky scrutinised the available evidence showing a role for NP and troponins as diagnostic/screening tools and prognostic markers in cardio-oncology. Data exist to support the utility of NP as markers for the risk of cardiomyopathy and CV events in several clinical settings, including patients with breast cancer receiving anthracyclines with or without trastuzumab, patients with multiple myeloma receiving proteasome inhibitors, and childhood survivors exposed to cardiotoxic therapies.12-14 Similarly, troponins may have a role as diagnostic and prognostic markers in radiation-induced injury, predicting myocarditis risk with immune checkpoint inhibitor therapy, and in the emerging area of chimeric antigen receptor (CAR) T-cell therapies.12,15-17 Dr Ky explained that work is ongoing to better define the role of these biomarkers as prognostic and predictive tools in the cardio-oncology arena, alongside efforts to discover new mechanistic markers.
The Value of Biomarkers in the ABC Stroke and Bleeding Scores
Professor Lars Wallentin
The ABC (age, biomarkers, clinical factors) bleeding and stroke risk scores have shown good discriminative ability with better prediction of risk for stroke, major bleeding, and death than clinical scores. Prof Wallentin explained how the ABC scores were derived from two large clinical trial populations encompassing nearly 15,000 patients from ARISTOTLE and over 8,500 from RE-LY.18,19 The strongest incremental biomarkers for stroke risk were found to be NT-proBNP and troponin, while growth-differentiation factor-15 (GDF-15) and troponin were the most important biomarker variables in predicting major bleeding risk (Figure 1).20-22 In all instances, biomarkers outperformed key clinical variables, such as age, in predicting risk. These ABC risk scores have also been validated in several large cohorts, including the ENGAGE AF-TIMI-48 trial, and were shown to be well calibrated, providing a reliable estimate of absolute event rate in AF patients on anticoagulants.21-23 Collectively, these ABC scores can be used to identify patients with different risk profiles and tailor treatment accordingly, for example pinpointing those most likely to benefit from newer anticoagulants such as apixaban over warfarin. Prospective testing to determine whether ABC score-guided treatment improves stroke-free survival is ongoing in the ABC-AF study.24 This prospective, randomised study will enrol approximately 6,500 AF patients and employ a primary endpoint of composite of stroke or death. Prof Wallentin concluded that ABC-AF risk scores could ultimately improve risk stratification and decision support in AF, helping cardiologists to better balance the risks and consequences of ischaemic events, bleeding, and mortality with different treatment strategies.
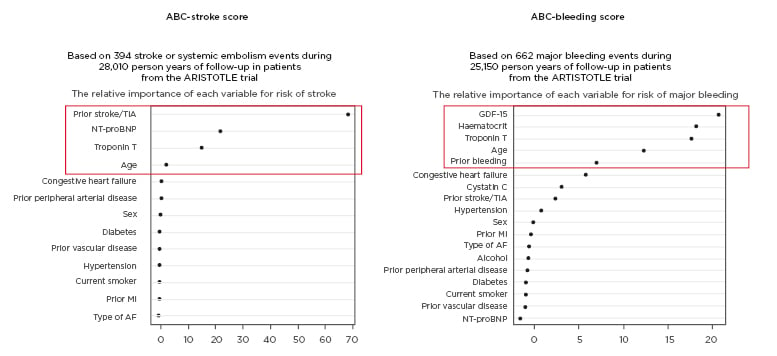
Figure 1: Relative importance of key biomarkers in the ABC-stroke and ABC-bleeding score in atrial fibrillation. AF: atrial fibrillation; GDF-15: growth-differentiation factor 15; MI: myocardial infarction; NT-proBNP: N-terminal B-type natriuretic peptide; TIA: transient ischaemic attack. Adapted from Hijazi et al.,20 Hijazi et al.,21 and Oldgren et al.22
The Role of Cardiac Biomarkers in Patients with COVID-19
Doctor Raphael Twerenbold
Coronavirus disease (COVID-19) is a systemic disease that can affect the heart, with cardiac manifestations resulting either from direct myocardial injury or viral-induced hypoxaemia/hyperinflammation.25 Data collected to date show that abnormalities in cardiac biomarkers occur frequently in patients with severe COVID-19 and are linked to adverse outcomes.26 In particular, hs-cTn and NT-proBNP have been shown to be powerful prognostic markers in COVID-19, irrespective of underlying CVD, and may have a predictive role in identifying more severe disease.
In a study of 187 hospitalised patients, mortality rates were 69.4% in patients with elevated cardiac troponin and underlying CVD, compared with 13.3% in patients with pre-existing CVD with normal troponin levels.27 The timing of troponin rise also appears to be significant, with hs-cTn increasing rapidly from Day 16 after disease onset in those with fatal outcomes.28 NT-proBNP is another key biomarker linked to severe/critical COVID-19.29 In a recent study, NT-proBNP showed strong discriminative incremental power to differentiate in-hospital death outcomes in COVID-19-positive patients (area under the curve: 0.909; 95% CI: 0.799–0.970; p<0.001), even after adjustment for other confounders.30 Currently, routine testing for cardiac biomarkers in patients with COVID-19 is not recommended by either the ESC or American College of Cardiology (ACC). However, given the important prognostic information embedded within these biomarkers and the clinical need to allocate the limited healthcare resources during the COVID-19 pandemic, Dr Twerenbold suggested that their measurement may be used to guide early triage and subsequent management in COVID-19. As such, he uses these biomarkers in patients with COVID-19 in his own clinical practice.
Cardiovascular Risk Assessment in Type 2 Diabetes Mellitus
Professor James Januzzi
A substantial body of evidence supports NT-proBNP as a versatile biomarker with a broad range of indications, including prediction of CV risk in patients with and without Type 2 diabetes mellitus (T2DM) in some clinical guidelines. Being in the midst of a global T2DM pandemic increases the risk for cardiorenal events including HF, said Prof Januzzi. Studies have shown that NT-proBNP is superior, and additive, to albuminuria in predicting CV events in T2DM, with serial measurements providing greater insight into incident HF risk than single spot measurements. Prof Januzzi gave a deep dive into newly generated data from the placebo-controlled CANVAS study of the sodium glucose cotransporter-2 (SGLT2) inhibitor canagliflozin, which evaluated multiple biomarkers in samples from 4,330 patients with T2DM and CV risk.31 Baseline NT-proBNP ≥125 pg/mL proved prognostic across every CV and kidney endpoint when analysed in adjusted models.31 Treatment with canagliflozin also appeared to blunt NT-proBNP rises over time, with a 12% lower increase on aggregate by Year 1 versus placebo.31,32 Other biomarkers may add to this ‘toolbox’ for cardiorenal risk prediction in T2DM, notably hs-cTnT, soluble isoform of ST2, and insulin-like growth factor binding protein 7 (IGFBP7) baseline levels, all of which were associated with adverse cardiac outcomes in CANVAS.33 Prof Januzzi described elevated concentrations of the senescence biomarker IGFBP7 as being associated with “remarkably high risk for incident HF,” with an HR of 14.2 for HF hospitalisation in the CANVAS study.Baseline IGFBP7 also predicted the benefit of canagliflozin on proteinuria progression, marking a potential step towards the ‘holy grail’ of using biomarkers to identify specific benefit from therapy.34 Moving forward, the ongoing PONTIAC II randomised trial will use baseline NT-proBNP levels to select intensified treatment for T2DM patients with the goal of improving outcomes in those at highest risk of HF.35
Multiplex Biomarkers: How Do They Compare to GDF-15 and NT-proBNP?
Doctor Agneta Siegbahn
Multiplex analysis provides a novel tool for biomarker discovery with the ability to identify protein signatures, improve the precision of diagnostics, and tailor treatment for individual patients. Dr Siegbahn outlined how new analytical high-throughput technologies, such as modified aptamers and proximity extension assays, allow for the simultaneous measurement of hundreds of biomarkers in a small volume of plasma. The PIVUS and USLAM population-based studies identified nine new risk markers of plaque prevalence and vulnerability using a proteomic chip proximity extension assay and the CVD1 biomarker panel, with NT-proBNP found to have the strongest predictive significance.36,37 In a further case-controlled study of multiplex screening for the association of CV and inflammatory biomarkers with CV death in patients with stable coronary heart disease, 18 biomarkers were confirmed across both derivation and validation cohorts. Of these, NT-proBNP, hsTnT, and GDF-15 were found to be the most powerful, outperforming >150 other biomarkers that were analysed simultaneously.38 In the area of AF, a multiplex proteomic chip approach has been used to explore pathophysiological pathways for incident AF in plasma samples from 1,694 individuals.39 Only five biomarkers retained a significant association after adjustment for clinical factors, of which by far the strongest was NT-proBNP (HR: 1.80; CI: 1.58–2.04; p=1.2×10-19).39
The Digital Revolution: Towards a Cardiovascular Algorithm Ecosystem
Doctor Rick Pleijhuis
Although thousands of CV risk models have been published in the literature, their actual implementation in clinical practice requires creation, validation, and integration of functional risk calculators. Dr Pleijhuis introduced Evidencio (Haaksbergen, the Netherlands), an algorithm ecosystem that aims to bridge the current gap between scientific literature and the clinical application of CV risk prediction models.40 Evidencio consists of a centralised cloud-based platform containing over 1,000 functional risk calculators hosted in a public library. In the model creation stage, standardised digital calculators are developed using inputs from published literature or statistical software. A user-friendly graphical interface is then generated instantaneously. Model validation is semi-automated based on local anonymised patient data in the target population. Dr Pleijhuis explained that the platform automatically produces data on model performance, including discrimination and calibration, and allows for direct head-to-head comparisons so physicians can select the best performing risk calculator. Single integration of the information technology infrastructure affords direct access to hundreds of prediction models simultaneously that can be integrated directly into the clinical workflow. With the European Union’s (EU) Medical Device Regulation (MDR) on the horizon in May 2021, the Evidencio platform can also help researchers and healthcare professionals in complying with relevant rules and regulations regarding certification of prediction models for clinical decision support.41
Perioperative Myocardial Infarction in Noncardiac Surgery
Professor Philip J. (PJ) Devereaux
Prof Devereaux presented compelling evidence from the VISION study, highlighting the clear link between troponin elevations after major noncardiac surgery, which is carried out in over 200 million patients annually, and increased risk of both 30-day mortality and 2-year major vascular events. VISION was a prospective cohort study of more than 40,000 representative patients ≥45 years old undergoing noncardiac surgery at 28 centres across 14 countries. In this study, patients with elevated cTnT ≥0.04 ng/L but with no ischaemic clinical features or ECG changes were found to have over a three-fold increase in the probability of death within 30 days (adjusted HR: 3.3; 95% CI: 2.26–4.81).42 This led to the development of diagnostic criteria for myocardial injury after noncardiac surgery (MINS). MINS is defined as myocardial infarction and isolated troponin elevations that occur within 30 days of noncardiac surgery without the need for ischaemic features (e.g., ischaemic symptoms or ECG findings), and without evidence of a nonischaemic aetiology (e.g., sepsis, pulmonary embolism).42 Similar results were seen with hs-cTnT, measured in around 21,000 VISION patients, where rising peak levels after noncardiac surgery were associated with an increasing probability of death, culminating in a 30% overall risk of death for patients at or above the highest risk threshold, 1,000 ng/L (adjusted HR: 227; 95% CI: 87.35–589.92; p<0.001).43 Prof Devereaux cautioned that without perioperative troponin measurements, >90% of MINS cases and >50% of myocardial infarctions will go undetected and defined MINS either as elevated postoperative hs-cTnT of 20 to <65 ng/L with an absolute change of ≥5 ng/L or hs-cTnT ≥65 ng/L. He therefore recommended measuring troponin before surgery and for the first 2 days thereafter to capture >95% of MINS, which, once identified, can then be effectively treated with dabigatran, aspirin, and/or statin therapy.
Artificial Intelligence-Based Combined Imaging and Circulating Biomarker Panels for Heart Failure
Professor Carolyn Lam
Missed diagnoses remain a significant problem in HF, particularly in primary care, with one in six individuals aged over 65 years with breathlessness estimated to have unrecognised HF.44 A key reason for this underdiagnosis is that NT-proBNP and echocardiography, the two critical cornerstones of objective HF diagnosis, can prove difficult to apply and complex to interpret in the primary care setting. As a diagnostic tool, echocardiogram is therefore suitable for artificial intelligence (AI) disruption. In a landmark 2018 paper, machines were taught to recognise the many views and variations on echocardiogram and achieved an average accuracy of 91.7%, compared with 79.4% for board-certified echocardiographers.45
From view recognition, it is then only a small step to AI-based segmentation, measurement, and interpretation, remarked Prof Lam. In a recent study, video-based AI demonstrated the ability to carry out beat-to-beat assessment, reliably classifying HF with reduced ejection fraction with an area under the curve of 0.97 despite limited human supervision.46 In the future, Prof Lam suggested that AI may even be used to guide ultrasound image capture, with patients performing their own echocardiograms, as directed by instructions from the computer in the ultimate ‘medical selfie’. For improved HF diagnosis, combining echocardiogram with circulating biomarkers and AI is likely to offer the ideal diagnostic panel. The ‘moonshot’ dream would then be to marry point-of-care echocardiograms assisted by AI with point-of-care NT-proBNP testing to give an automatic, instantaneous determination of the probability of HF in an individual patient.
Use of Biomarkers to Identify Therapy Responders in Heart Failure
Doctor Nasrien Ibrahim
The four established pillars of survival-enhancing medical therapy for HF with reduced ejection fraction (angiotensin receptor-neprilysin inhibitors, aldosterone receptor antagonists, β-blockers, and SGLT2 inhibitors) may soon be joined by newer modalities such as omecamtiv mecarbil and vericiguat. Dr Ibrahim explained that, as the HF armoury continues to expand, biomarkers will have a key role in elucidating underlying mechanisms of drug efficacy and identifying therapy response, marking an important first step towards personalised treatment. Cardiac reverse remodelling is an important therapeutic mechanism that has been linked to improved CV outcomes.47 NT-proBNP, the gold standard cardiac biomarker, may inform cardiac remodelling by reflecting myocardial stretch and neurohormonal activation. In clinical trials, NT-proBNP was found to be associated with left ventricular remodelling, with NT-proBNP responders showing a greater degree of reverse remodelling.48,49 In the PROVE-HF study of sacubitril/valsartan, NT-proBNP dropped rapidly and significantly in the first 2 weeks and remained low throughout the 1-year trial period.49 Reverse cardiac remodelling was also sustained for the full 12 months, with ongoing increases in left ventricular ejection fraction (LVEF) over the course of 1 year. The overall improvement in LVEF was +9.4% at 12 months and one-quarter of patients achieved a LVEF increase of ≥13% at this timepoint.50 Subgroup analysis revealed that females reverse remodelled faster than males, and patients who were black, despite starting out with the lowest LVEF, experienced comparable reverse remodelling to other ethnic groups.51 Notably, patients with larger and faster reduction in NT-proBNP and left ventricular end-systolic volume index by 6 months also had the lowest rates of subsequent death or HF hospitalisation by 12 months (Figure 2).52
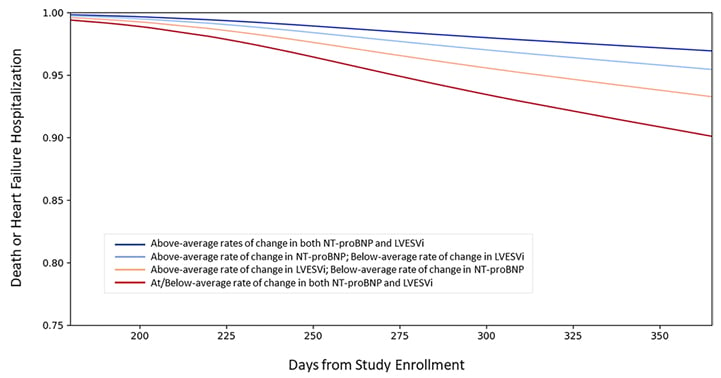
Figure 2: Link between N-terminal B-type natriuretic peptide, left ventricular end-systolic volume index, and risk of death or heart failure hospitalisation. LVESVi: left ventricular end-systolic volume index; NT-proBNP: N-terminal B-type natriuretic peptide. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health, Inc. Please contact [email protected] for further information. Adapted from Januzzi et al.52
Similar findings have been seen with other NP when exploring how neprilysin inhibition leads to therapeutic benefit. In the PROVE-HF trial, early and significant increases in atrial NP were correlated with cyclic GMP change and larger rises in atrial NP were predictive of greater gains in LVEF and larger reduction of left atrial volume index.53 Collectively, this accumulated evidence suggests that biomarkers such as NT-proBNP may help to inform treatment response in HF by providing an important anchor to the underlying myocardial biology.
Interactive Question and Answer Session
Each of the 3 days of the proCardio webinar concluded with an interactive question and answer session involving all speakers, with questions posed by the audience. These sessions fuelled some engaging debate and discussion among the assembled experts, as well as providing a valuable opportunity to deep dive into the key data presented.
As in previous years, proCardio 2020 proved an engaging and informative event, providing important updates and novel insights into the field of biomarkers in CV medicine. New data across the domains of AF, coronary heart disease, and HF continue to reinforce the established and emerging role of biomarkers in routine clinical practice.

![EMJ Cardiology 9 [Supplement 2] 2021 feature image](https://www.emjreviews.com/wp-content/uploads/2021/03/EMJ-Cardiology-9-Supplement-2-2021-feature-image-940x563.jpg)






