Presenters: Sheila Hegde,1 Sara Saberi2
1. Brigham and Women’s Hospital, Boston, Massachusetts, USA
2. University of Michigan Medical School, Ann Arbor, Michigan, USA
Disclosure: Dr Hegde has received an institutional grant from MyoKardia. Dr Saberi has received consulting fees from MyoKardia.
Acknowledgements: Writing assistance was provided by Amanda Barrell, Brighton, UK.
Support: The writing and publication of this article was funded by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers SquibbTM. The EXPLORER-HCM study was funded by MyoKardia, Inc., a wholly owned subsidiary of Bristol Myers SquibbTM.
Citation: EMJ Cardiol. 2021;9[Suppl 1]:2-6.
Meeting Summary
Obstructive hypertrophic cardiomyopathy (HCM) is a progressive myocardial disorder characterised by dynamic left ventricular outflow tract (LVOT) obstruction resulting from ventricular hypertrophy and mitral valve systolic anterior motion (SAM), and by elevated left ventricular (LV) filling pressures.1 It is a disease of cardiac sarcomere proteins, in which excess myosin–actin cross-bridging drives hypercontractility, impaired relaxation and compliance, and increased energetic burden.2 Obstructive HCM can be a debilitating disease that results in impaired functionality and lower quality of life, and adverse cardiac remodelling can lead to a number of serious complications, including atrial fibrillation and heart failure.
Mavacamten, a first-in-class selective cardiac myosin inhibitor, works by targeting the underlying pathology of HCM and reducing the number of myosin–actin cross-bridges. The Phase III EXPLORER-HCM study found that treatment with mavacamten led to improvement in symptoms, function and exercise capacity, LVOT gradients, and health status.3 Analyses of data from a cardiac imaging substudy that used echocardiography and cardiac magnetic resonance (CMR) provided additional information on the effect of mavacamten on cardiac structure and function.4 Both studies are summarised below.
Mavacamten Favourably Impacts Key Pathophysiologic Processes in Obstructive Hypertrophic Cardiomyopathy: Results from the EXPLORER-HCM Study
Doctor Sheila Hedge
As the highest ranked abstract submitted to the meeting from the USA, this ePoster5 received the 2020 Paul Dudley White International Scholar Award.
Background
Current pharmacologic management of HCM includes β-blockers, nondihydropyridine calcium channel blockers, and disopyramide. However, patients often experience symptoms, including chest pain, fatigue, and arrhythmias, despite being on therapy.6
The pivotal Phase III EXPLORER-HCM study set out to evaluate mavacamten’s effect on focussed echocardiographic measures of cardiac structure and function.
Methods
EXPLORER-HCM7 was an international, Phase III, multicentre, randomised, double-blind, placebo-controlled study of mavacamten in patients with symptomatic obstructive HCM.
Researchers enrolled 251 patients with a resting and/or provoked LVOT gradient ≥50 mmHg, LV ejection fraction (EF) ≥55%, and New York Heart Association (NYHA) Class II or III symptoms. Patients were randomised 1:1 to mavacamten (n=123) or placebo (n=128) over a period of 30 weeks. Patients in the treatment group received a starting dose of 5 mg per day.
Exploratory endpoints included a change from baseline to Week 30 in echocardiographic parameters of LV and left atrial (LA) structure and function, particularly LV mass index, LA volume index, e’ velocities, and E/e’. The presence or absence of the SAM of the mitral valve and mitral regurgitation (MR) were also assessed.
Echocardiograms were performed every 2–4 weeks for safety, with select echocardiography measures taken at screening, Week 18, and Week 30. Image analysis was performed by the Cardiovascular Imaging Core Laboratory at Brigham and Women’s Hospital, Boston, Massachusetts, USA, in accordance with American Society of Echocardiography (ASE) recommendations.
Results
On average, participants were 59 years old. Overall, 41% were female and 91% were white. Just under one-third, 27%, had a family history of HCM and 8% had a history of septal reduction. Three-quarters were on background β-blocker therapy, and 17% were taking calcium channel blockers.
At baseline, patients exhibited typical features of obstructive HCM, including hyperdynamic LV systolic function and increased wall thickness. Increased LVOT gradients were observed at rest, with Valsalva, and postexercise. Patients also demonstrated mildly dilated LA volumes, reduced e’ velocities, and elevated E/e’ ratios, all consistent with abnormal diastolic function. Most had SAM and MR, but researchers were unable to assess right ventricular systolic pressure in most patients.
When compared with placebo, mavacamten treatment for 30 weeks led to significant improvements in LV mass index, LA volume index, lateral e’, and lateral E/e (Table 1).
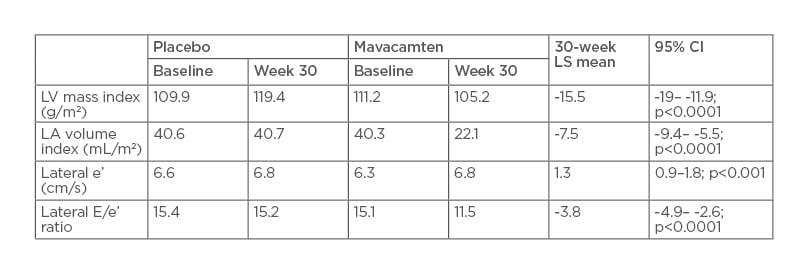
Table 1: Change in echocardiographic parameters.
CI: confidence interval; LA: left atrium; LS: least squares; LV: left ventricle.
Significantly more patients receiving mavacamten than placebo achieved SAM resolution at Week 30 (80.9% versus 34.0%). MR resolved in 9.0% of treated patients, but none of the placebo group.
Conclusions
Treatment with mavacamten resulted in significant improvements in LV relaxation, shown by an increase in e’ velocity, leading to improvements in LV filling pressures, shown by decreased E/e’ ratios. Furthermore, significant resolution of SAM with previously reported reductions in LVOT obstruction suggest mitigation of hypercontractility while maintaining normal LVEF. Extension studies are ongoing to determine the long-term effect of mavacamten on these adverse pathophysiologic processes that are hallmarks of obstructive HCM.
Mavacamten Favourably Impacts Cardiac Structure in Obstructive Hypertrophic Cardiomyopathy: EXPLORER-HCM Cardiac MRI Substudy
Doctor Sara Saberi
This CMR substudy of the EXPLORER-HCM trial demonstrated favourable effects of mavacamten on cardiac structure and function in obstructive HCM after 30 weeks of treatment.4
Eligibility criteria included being aged 18 years or older with symptomatic outflow obstruction and normal LV systolic function. Patients had to be free of implanted cardiac devices and pacemakers and have no evidence of atrial fibrillation at screening.
On Day 1, patients were randomised 1:1 to either mavacamten at 5 mg per day or placebo and underwent CMR. A second CMR was carried out at Week 30. At Weeks 8 and 14, researchers were able to adjust mavacamten dosage, dependent on pharmacokinetic parameters, the degree of outflow obstruction, and EF.
The primary endpoint was change in LV mass index from baseline to Week 30. Exploratory endpoints included other markers of ventricular hypertrophy and cardiac structure, including absolute myocardial mass index, maximum wall thickness, LV function and volumes, LA volumes, and myocardial fibrosis.
Results
Thirty-five EXPLORER-HCM trial participants from 15 sites took part in the substudy. Mean age was 60 years and 43% of patients were female. Demographic and baseline characteristics were generally well balanced between the treatment (n=17) and control (n=18) groups; although, there was a trend towards longer duration of HCM in the placebo cohort (8 years versus 5 years). Most patients had NYHA Class II symptoms (76.5% in the mavacamten group and 72.2% in the placebo group) and the majority were also on background therapy, most frequently β-blockers.
Both groups had significant hypertrophy burden, with maximum wall thickness nearly twice normal levels, and elevated LV mass index at baseline.
The study recorded a mean 17.4% reduction in LV mass index in the mavacamten group compared with a 1.6% reduction in the placebo group. Treated patients also saw greater reductions in absolute myocardial mass index, global LV maximum thickness, and maximum septum wall thickness.
After 30 weeks of treatment with mavacamten, reductions in primary and exploratory endpoints of LV hypertrophy and LA volumes were observed (Table 2). As expected, there was a small reduction in LVEF, and all patients remained in the normal range.
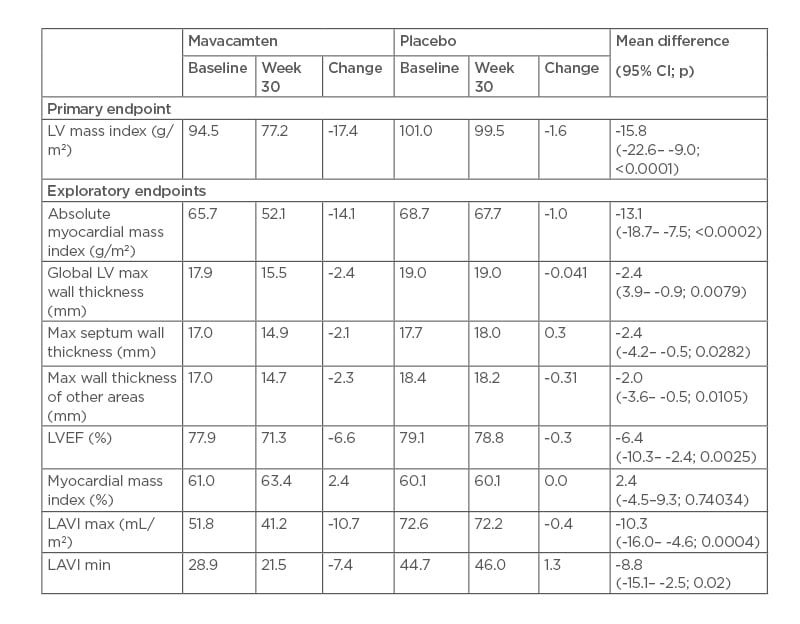
Table 2: Left ventricle mass and wall thickness, and cardiac function and volume.
CI: confidence interval; LAVI: left atrial volume index; LV: left ventricle; LVEF: left ventricular ejection fraction; max: maximum; min: minimum.
Myocardial contraction fraction, a volumetric assessment of LV systolic function, and the stroke volume to LV mass ratio did not change with mavacamten, reaffirming the overall normal LV systolic function with treatment.
There were low levels of interstitial fibrosis at baseline, an important prognostic factor in HCM. After 30 weeks of treatment with mavacamten, there was no worsening in fibrosis, as confirmed by no changes in gadolinium enhancements or extracellular volume fraction. Changes in LV mass index positively correlated with a decline in high-sensitivity cardiac troponin (Rho=0.75; 95% confidence interval: 0.53–0.87) in both treatment groups, which warrants further investigation.
Conclusion
Treatment with mavacamten for 30 weeks was associated with favourable cardiac remodelling in patients with obstructive HCM. Observed reductions in LV hypertrophy and LA volumes were not associated with increased fibrosis, and contractile function remained within normal ranges.
This was the first randomised controlled study to show a favourable impact of a pharmacological agent on cardiac structure in HCM using CMR. The work also demonstrated that CMR is a sensitive assessor of disease progression. The long-term impact of mavacamten on cardiac structure and function is being investigated in a 5-year long-term extension study.








