Meeting Summary
Prof Masana presented evidence that low-density lipoprotein (LDL) cholesterol is a causal factor for atherosclerosis and that cardiovascular disease (CVD)-relative risk (RR) is reduced proportionally to LDL reductions, regardless of the type of monotherapy used. Combination therapy offers the advantage of increased lipid-lowering efficacy and a reduction in the side effects associated with high-intensity statins. The rationale thus exists for replacing high-intensity statin therapy with high-intensity cholesterol-lowering therapy.
Prof Farnier gave an in-depth description of the results of the IMPROVE-IT, FOURIER, and ODYSSEY-Outcomes trials, demonstrating that the magnitude of clinical benefit is independent of whether it is achieved by statins, ezetimibe, or PCSK9 inhibitors. The IMPROVE-IT study also showed that the magnitude of benefit is proportionate to the absolute decrease in LDL cholesterol. This is consistent with the conclusions of a meta-analysis of randomised controlled statin trials, showing that patients achieving very low LDL cholesterol levels have a reduced risk of major cardiovascular (CV) events compared with those achieving moderately low levels. The greatest benefits for reductions in major adverse CV events from lowering LDL cholesterol occur in patients with diabetes.
The above studies have led the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) taskforce on PCSK9 inhibitors to outline a strategy for additional treatment, with patients on maximally-tolerated statin doses failing to achieve LDL cholesterol goals at 4 weeks being considered for ezetimibe treatment, and those failing to achieve goals after a further 4 weeks being considered for PCSK9 inhibitors.
Votes from the audience, collected at the start and end of each presentation, showed that the speakers convinced delegates that the lower the LDL cholesterol level achieved the better the outcome for patients would be, that combination therapy is as effective as single dose high-intensity statins, and that statins plus ezetimibe should be considered as standard treatment in high-risk patients, particularly in Type II diabetes mellitus (T2DM) patients.
From Guidelines to Real Life: Practical Considerations for Patients with Hypercholesterolaemia. Combination Therapy
Professor Lluis Masana
The audience were asked to consider the case of a 71-year-old ex-smoker with hypertension treated with angiotensin-converting-enzyme inhibitors, calcium inhibitors, and hydrochlorothiazide, who was admitted to hospital with acute myocardial infarction (MI). The patient had untreated moderate hypercholesterolaemia (total cholesterol: 213 mg/dL; high-density lipoprotein [HDL] cholesterol: 43 mg/dL; LDL cholesterol: 140 mg/dL; and triglycerides [TG]: 150 mg/dL) and had been prescribed omeprazole.
In the first vote, 62%, 4%, and 6% of delegates said LDL cholesterol therapy targets should be <70 mg/dL, <50 mg/dL, and <30 mg/dL, respectively (NB. 1 mg/dL cholesterol=0.02586 mmol/L). Eight percent said that a 50% reduction should be achieved, 8% indicated that no target was necessary if a high-intensity statin was used, and 11% said that the lowest possible LDL cholesterol level should be aimed for. The LDL cholesterol target for such a patient is <70 mg/dL.1
In the second vote, 51% and 23% of delegates indicated that the patient should be started on atorvastatin 80 mg or rosuvastatin 20 mg, respectively. Fourteen percent said they would start on atorvastatin 40 mg/ezetimibe 10 mg, 3% voted for atorvastatin 80 mg/ezetimibe 10 mg, 1% decided on rosuvastatin 10 mg/ezetimibe 10 mg, and 7% chose rosuvastatin 20 mg/ezetimibe 10 mg.
Such responses demonstrated that three-quarters of the audience were aware of the ESC/EAS and the American College of Cardiology (ACC)/American Heart Association (AHA) 2013 guidelines on blood cholesterol treatment recommending that hypercholesterolaemic patients who have experienced a MI should receive high-intensity statins before being considered for combination therapy. For patients at higher risk for side effects, moderate-intensity statins can be started and, if this fails, statins should be increased to the highest intensity tolerable; only if this approach does not work should combination therapy be considered.1,2
Guidelines were developed after several trials showed no CV benefits for non-statin lipid-modifying agents used in combination with statins versus statin monotherapy. Negative trials included ILLUMINATE (torcetrapib),3 HPS2-THRIVE (niacin),4 DAL-OUTCOMES (dalcetrapib),5 AIM-HIGH (niacin),6 and ACCELERATE (evacetrapib).7 Positive outcomes were observed in two studies, FIELD8 and ACCORD,9 for the fenofibrate/statin combination in patient subsets with high TG and low HDL cholesterol but not in overall diabetic subjects. Following these findings, the AHA and ACC 2013 cholesterol treatment guidelines recommended high-intensity statins as the preferred option for established CVD or hyperlipidaemia.2
Notably, in 2017, a EAS comprehensive review of scientific studies concluded that LDL cholesterol is not just a CV risk biomarker but also an aetiological factor in atherosclerosis.10
The LDL cholesterol level associated with CV outcomes has been established by a meta-regression analysis for statin and non-statin therapies.11 The analysis included statins (25 trials), fibrates (9 trials), ezetimibe (1 trial), niacin (3 trials), cholesteryl ester transfer protein inhibitors (3 trials), diet (4 trials), bile acid sequestrants (2 trials), ileal bypass surgery (1 trial), and PCSK9 inhibitors (2 trials). The analysis found a 23% reduction in CV events for each mmol/L reduction in LDL cholesterol when patients were treated with statins (RR: 0.77; 95% confidence interval [CI]: 0.71–0.84; p<0.001). A similar RR was reported for five non-statin therapies, including diet, bile acid sequestrant, ileal bypass, and ezetimibe (RR: 0.77; 95% CI: 0.75–0.79; p<0.001). The findings indicated that the relationship between CV event risk and LDL cholesterol levels achieved exists for both statins and certain non-statin therapies.
The IMPROVE-IT study demonstrated a relationship between incremental lowering of LDL cholesterol and improved CV outcomes when the non-statin lipid-modifying agent ezetimibe was added to simvastatin.12 This suggests that, regardless of the mechanism of action, all reductions in LDL levels are of equivalent benefit and that a high-intensity cholesterol-lowering strategy should replace high-intensity statin therapy.13
Current cholesterol-lowering drugs include statins, ezetimibe, PCSK9 inhibitors, and resins/colesevelam. TG-lowering drugs include fibrates and omega-3 fatty acids. In addition to each agent reducing CV events as a monotherapy, there is now evidence supporting the effectiveness of combining statins with ezetimibe and PCSK9 inhibitors. There is also evidence supported by post-hoc analyses that statins and fenofibrate may be useful in patients with diabetes and atherogenic dyslipidaemia. Negative results have been reported for statin–omega 3 combinations, although high-dose omega 3 fatty acids with fibrates are used to treat patients with very high TG levels.1
Cholesterol-lowering therapies are classified into four groups according to LDL cholesterol reduction intensity, with low, mild, high, and very-high-intensity cholesterol-lowering therapies reducing LDL cholesterol by <30%, 30–49%, 50–60%, and >60%, respectively.13 Very-high-intensity combinations include atorvastatin 40–80 mg/ezetimibe 10 mg and rosuvastatin 20–40 mg/ezetimibe 10 mg.12
Since 1984, randomised controlled trials have achieved progressively lower LDL cholesterol concentrations. For example, the latest FOURIER study reported LDL levels of 30 mg/dL.14 A pre-specified secondary analysis of this study demonstrated that such a concentration is more favourable, in terms of CV outcomes, than concentrations of 90 mg/dL or 70 mg/dL.15
While these data suggest that CV prevention is improved with lower LDL cholesterol levels, only 28.1% of CVD patients reach LDL cholesterol targets, according to the DYSIS targets.16
DYSIS furthermore found that, in patients at very high CV risk, the mean difference between actual levels and target levels was roughly 1 mmol/L, and that for populations failing to achieve LDL cholesterol targets the mean starting dose was equivalent to 35 mg/day of simvastatin, and only 7% received combination therapy. Such data suggests the need to address undertreatment.
Treatment options for patients taking medium or standard doses of statins who are not exhibiting the desired response include doubling their dose, switching to a more powerful statin, and starting combination therapy.1
A study that compared LDL cholesterol reductions for a range of statins (e.g., atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatin, and rosuvastatin) and a second study looking at pitavastatin found that only atorvastatin and rosuvastatin at daily doses of ≥20 mg produced reductions >40%.17,18 Furthermore, when the atorvastatin dose was doubled, from 40 mg to 80 mg, the LDL lipid-lowering effect increased by only 6%, from 44% to 50%. Reductions in LDL cholesterol achieved from doubling doses are less pronounced than those observed following the addition of other agents. Combining two different lipid-lowering drugs provides a greater LDL cholesterol decrease; however, this does not simply correspond to the addition of each individual drug effect. For example, rosuvastatin 20 mg/day and ezetimibe 10 mg/day lowered LDL by 50% and 20%, respectively, but the two agents combined lower LDL cholesterol by 60%.19
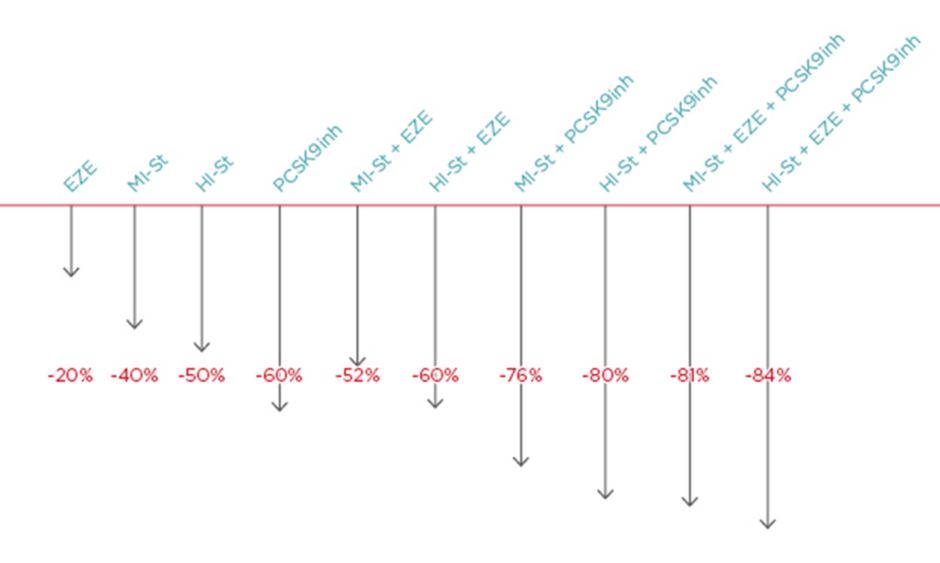
Generally, the following LDL cholesterol reductions can be achieved with therapy: ezetimibe: -20%; moderate-intensity statin: -40%; high-intensity statin: -50%; PCSK9 inhibitor: -60%; high-intensity statin plus ezetimibe: -60%; moderate intensity statin plus PCSK9 inhibitor: -76%; high-intensity statin plus PCSK9 inhibitor: -80%; moderate-intensity statin plus ezetimibe plus PCSK9 inhibitor: -81%; and high-intensity statin plus ezetimibe plus PCSK9 inhibitor: -84%.20 Thus, maximum LCL cholesterol lipid lowering can be achieved with combinations of high intensity statins plus ezetimibe plus PCSK9 inhibitors. Notably, the difference between high-intensity and moderate-intensity statins when combined with ezetimibe and PCSK9 inhibitors is just 3% (Figure 1).19
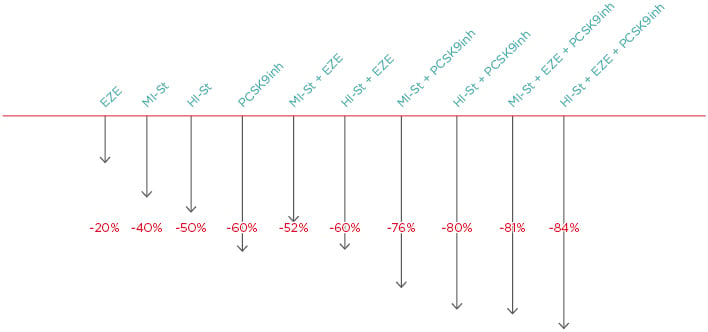
Figure 1: The lipid-lowering difference between high-intensity and moderate-intensity statins when combined with ezetimibe and PCSK9 is 3%.
EZE: ezetimibe; HI-St: high-intensity statin; inh: inhibitor; MI-St: moderate-intensity statin.
Adapted from Masana et al.19
Benefits of statins, including CVD reduction and increased survival, need to be balanced against risks such as developing muscle symptoms, T2DM, and transaminases elevation. Evidence from studies, including PROVE-IT, A-Z, TNT, IDEAL, and SEARCH, suggests that the diabetogenic effects of statins are dose- related.21 According to a EAS panel, factors influencing the risk of statin-associated muscle symptoms include pre-existing risk factors and comorbidities, high-dose statin therapy, polypharmacy, and drug–drug interactions (including concomitant use of gemfibrozil, macrolides, azole antifungal agents, protease inhibitors, and immunosuppressive drugs such as cyclosporine and inhibitors of CYP450 isoenzymes).22
To conclude, LDL cholesterol can be considered a causal factor for atherosclerosis, with the RR of CVD reduced proportionally to LDL cholesterol decreases regardless of the therapy used. The benefits of combination therapy, such as statins plus ezetimibe and statins plus PCSK9 inhibitors, have a scientific evidence base. Combination therapy increases lipid-lowering efficacy and reduces side effects associated with high-intensity statins, improving adherence and CV event reduction. Thus, the rationale exists for replacing high-intensity statin therapy with high-intensity cholesterol-lowering therapy.
In a repeat of the original vote, 39%, 4%, and 0% of the audience said LDL cholesterol therapy targets should be <70 mg/dL, <50 mg/dL, and <30 mg/dL, respectively. Eleven percent said that a 50% reduction should be achieved, 17% indicated there is no target when high intensity statins are used, and 29% that the lowest possible level should be aimed for.
Regarding the second vote on treatments, 51% choose atorvastatin 80 mg, 14% voted for rosuvastatin 20 mg, 20% for atorvastatin 40 mg plus ezetimibe, 10% for atorvastatin 80 mg plus ezetimibe, 5% for rosuvastatin 10 mg plus ezetimibe, and 15% for rosuvastatin 20 mg plus ezetimibe.
The feedback from the audience demonstrated that the presentation had been successful in convincing delegates that the lower the LDL cholesterol level achieved the more favourable the outcome for patients and that use of combination therapy was as effective as single-dose high-intensity statins.
Interpretation of Recent Trial Outcomes and Considerations for Daily Practice
Professor Michel Farnier
At the start of Prof Farnier’s presentation, the audience was asked to consider the case of a 62-year-old male with T2DM treated with coronary artery bypass graft who had a lipid profile on rosuvastatin 20 mg of an LDL cholesterol level of 81 mg/dL, TG levels of 180 mg/dL, and HDL-C levels of 40 mg/dL.
In the first vote, 6% of the delegates opted for no change to treatment, 27% for increasing the dose of rosuvastatin to 40 mg, 55% for adding ezetimibe, 4% for adding a PCSK9 inhibitor, and 7% for adding fenofibrate.
A number of trials have shown benefits from adding LDL therapies to statins, including IMPROVE-IT (ezetimibe),12 REVEAL (anacetrapib), FOURIER (evolocumab),15 SPIRE-1 and 2 (bococizumab),23 and ODYSSEY-Outcomes (alirocumab).24 Bococizumab and anacetrapib have since been withdrawn due to antidrug antibodies, which caused the LDL-lowering effect to wear off in bococizumab trials and accumulation in adipose tissue with anacetrapib.
In the IMPROVE-IT study, 18,144 patients with post-acute coronary syndrome for <10 days with LDL cholesterol of 50–100 mg/dL (on lipid-lowering therapy) or 50–125 mg/dL (without lipid-lowering therapy) were randomised to simvastatin 40 mg plus ezetimibe 10 mg or simvastatin 40 mg plus placebo.12 Results at a median follow-up of 6 years showed that the primary endpoint (CV death, MI, documented unstable angina requiring rehospitalisation, coronary revascularisation for >30 days, or stroke) occurred in 32.7% of patients in the simvastatin plus ezetimibe group versus 34.7% in the simvastatin plus placebo group (hazard ratio [HR]: 0.936; 95% CI: 0.89–0.99; p=0.016).12
In the FOURIER trial,25 27,564 stable patients with established CVD (prior MI [81%], prior stroke [19%], or symptomatic peripheral artery disease [PAD] [13%]), 69% of whom were on high-intensity statins, who had either LDL cholesterol >70 mg/dL or non-HDL cholesterol >100 mg/dL were randomised to evolocumab every 2 weeks (or every 4 weeks) versus placebo. At a median follow-up of 2.2 years the primary efficacy endpoint (CV death, MI, stroke, hospitalisation for unstable angina, or coronary revascularisation) occurred in 11.3% of patients taking placebo versus 9.8% taking evolocumab (HR: 0.85; 95% CI: 0.79–0.92; p<0.001). The RR reduction was 15% and absolute risk reduction 1.5%.
Finally, in the ODYSSEY Outcomes trial, 18,924 patients were randomised to alirocumab or placebo plus background high-intensity statins starting 1–12 months after acute coronary syndrome. All participants had baseline LDL cholesterol levels of ≥70 mg/dL despite intensive statin treatment. The specific strategy was to obtain LDL cholesterol targets between 25 and 50 mg/dL, with some subjects needing up-titration of alirocumab, others down-titration of alirocumab, and a few switching to placebo when cholesterol became too low (<15 mg/dL).
Results at a median of 2.8 years showed the primary endpoint (a composite of coronary heart disease death, nonfatal MI, ischaemic stroke, or unstable angina requiring hospitalisation) occurred in 9.5% of alirocumab patients versus 11.1% of placebo patients (HR: 0.85; 95% CI: 0.78–0.93; p=0.0003). The study was presented by Prof Gabriel Steg at the 2018 ACC meeting.24
Taking the IMPROVE-IT, FOURIER, and ODYSSEY-Outcomes studies together, the magnitude of LDL cholesterol lowering benefit is independent of whether it is achieved by statins, PCSK9 inhibitors, or ezetimibe. This conclusion is in alignment with studies showing genetic mutations affecting LDL metabolism that decrease LDL cholesterol results in dose-dependent decreases in atherosclerotic cardiovascular disease (ASCVD) risk.10 The magnitude of benefit is proportional to the absolute decrease in LDL cholesterol, which is demonstrated by the IMPROVE-IT study showing that reductions in LDL cholesterol are directly related to reductions in event rates.12 When the same durations of follow-up were used to compare PCSK9 inhibitors in the FOURIER and SPIRE trials with statin treatment in the Cholesterol Treatment Trialists meta-analysis of statin trials, a similar magnitude of risk reduction was obtained. At 1 year of treatment, for every 1 mmol/L of LDL cholesterol reduction, the RR reduction of major vascular events was 14% in SPIRE 2 and 12% in CTT with statins. At 2 years of treatment, for every 1 mmol/L of LDL cholesterol reduction, the RR reduction of major vascular events was 17% in FOURIER versus 17% in CTT with statins.26 Together with the genetic evidence, this demonstrates that therapies reduce CV event risks proportionally to absolute achieved reductions in LDL cholesterol and total durations of therapy. For non-statin therapies used for ASCVD, cost-effectiveness analyses are essential to determine whether, how, and when PCSK9 inhibitor treatment meets accepted value for financial metrics.27 The higher the risk, the higher the benefit, and this raises a key question of how to identify statin therapy patients at highest risk of recurrent disease for additional treatments.
A meta-analysis of eight randomised controlled statin trials demonstrated that patients achieving very low LDL cholesterol levels had lower risks of major CV events than those achieving moderately low levels. Compared to patients achieving LDL cholesterol >175 mg/dL, those reaching LDL cholesterol 75–100 mg/dL, 50–75 mg/dL, and <50 mg/dL had adjusted HR for major CV events of 0.56, 0.51, and 0.44, respectively.28
Similar relationships hold for events when the placebo arms in FOURIER and ODYSSEY-Outcomes were analysed. In FOURIER, 13.6% of patients with LDL cholesterol <80 mg/dL had a CV event versus 16.2% with LDL cholesterol >109 mg/dL. In the ODYSSEY-Outcomes trial, 9.5% of patients with LDL cholesterol <80 mg/dL experienced an event versus 14.9% with LDL cholesterol >100 mg/dL.
For the FOURIER placebo group, the risk of CV death, MI, or stroke was 10.8% for patients <2 years from qualifying MI versus 9.3% for patients >2 years from qualifying MI (HR: 1.19; 95% CI: 1.04–1.37; p=0.01). The risk changes to 15.0% for patients having over two prior MI versus 8.2% for patients having one prior MI (HR: 2.04; 95% CI: 1.78–2.35; p<0.001), and 12.6% for patients having multivessel disease versus 8.9% for single vessel disease (HR: 1.47; 95% CI: 1.27–1.70; p<0.001). Analysis of FOURIER placebo data showed that the risk of CV, MI, or stroke was 13.0% in patients with PAD versus 7.6% in patients without PAD (adjusted HR: 1.81; 95% CI: 1.53–2.14; p<0.001).
Among the categories of highest risk ASCVD on statin therapy (defined as around or above a benchmark of 30% of a 10-year risk) are patients with clinical atherosclerotic CVD and T2DM.29
When IMPROVE-IT was analysed according to diabetic status, in non-diabetic patients the probability of the primary endpoint at 7 years was 30.2% for patients taking simvastatin or ezetimibe versus 30.8% for patients taking simvastatin alone (HR: 0.98; 95% CI: 0.91–1.04; p=0.526). For diabetic patients it was 40.0% for patients taking simvastatin or ezetimibe versus 45.5% for patients taking simvastatin alone (HR: 0.85; 95% CI: 0.78–0.94; p<0.001).30 Such data demonstrate that patients with diabetes derived significantly greater relative and absolute benefits from the addition of ezetimibe than patients without diabetes.
A recent meta-analysis of seven trials showed the ezetimibe plus statin combination therapy was associated with a greater reduction in major adverse CV events in diabetic patients than those without diabetes (pooled RR: 0.84 versus 0.93; pheterogeneity=0.012).31 Such data raise questions about why ezetimibe delivers greater benefits to patients with T2DM. The benefit was shown in IMPROVE-IT, where there was a 3 mg/dL greater reduction in LDL cholesterol for patients with diabetes compared to those without (p=0.03).30 Furthermore, a pooled analysis of 27 clinical trials of patients with and without diabetes receiving ezetimibe plus statin or statin alone showed patients with diabetes achieved significantly larger reductions in LDL cholesterol with the combination therapy (difference of 2.5%; p<0.0001).32
Reductions in cholesterol do not fully explain the magnitude of the effect in diabetic patients, leading to suggestions that other beneficial effects may also occur, including influencing postprandial lipaemia (a condition in which TG-rich chylomicron remnants are increased during the postprandial period).33
The ESC and EAS recently updated their practical clinical guidance for the use of PCSK9 inhibitors in patients with ASCVD.34 The new guidelines recommend that in patients with clinical ASCVD, ezetimibe should be given according to clinical judgement and local guidance, and that for patients with LDL cholesterol >140 mg/dL or patients with LDL-C >100 mg/dL and additional indices of risk, a PCSK9 inhibitor should be considered. Additional risk categories include familial hypercholesterolaemia, diabetes with target organ damage or marked hypertension, severe or extensive ASCVD, and rapid progression of ASCVD.
The taskforce additionally provided a decision algorithm for PCSK9 inhibitors in familial hypercholesterolaemia in primary prevention, with patients who have no additional indices being considered for PCSK9 inhibitors when they have LDL cholesterol levels >180 mg/dL and for patients with additional indices of risk severity being considered with LDL cholesterol levels >140 mg/dL. Here, additional risk indices are defined as diabetes with target organ damage, lipoprotein (a) >50 mg/dL, major risk factors such as smoking, marked hypertension, >40 years of age without treatment, and premature ASCVD (<55 years in males and <60 years in females) in first-degree relatives and imaging indicators.
The taskforce recommendation is to first place patients on the maximally tolerated statin doses and assess their response at 4 weeks. Those failing to achieve LDL cholesterol goals should be considered for ezetimibe, with their response assessed again after 4 weeks. Those found to be above LDL cholesterol thresholds should then be considered for a PCSK9 inhibitor. Patients prescribed the PCSK9 inhibitors should have their LDL cholesterol-lowering response assessed 2 weeks after the first injection.
A recent analysis of the DYSIS II study found that, among 631 Italian patients with coronary heart disease receiving lipid lowering treatment, 64.6% had LDL cholesterol levels >70 mg/dL and 25.1% had LDL cholesterol levels >100 mg/dL. In a model, the addition of ezetimibe reduced the percentage of patients with LDL cholesterol >70 mg/dL and >100 mg/dL from 64.6–37.9% and from 25.1–11.8%, respectively.35
A simulation model involving a cohort of 105,269 patients was undertaken to estimate the percentage of patients with ASCVD that would require a PCSK9 inhibitor.36 The USA database revealed that only 25.2% achieved LDL cholesterol levels <70 mg/dL, with 51.5% of the database using statin monotherapy and 1.7% statins plus ezetimibe. The model showed that following treatment intensification, 99.3% could achieve LDL cholesterol levels <70 mg/dL including 67.3% with statin monotherapy, 18.7% with statins plus ezetimibe, and 14% with add-on PCSK9 inhibitors. In conclusion, combining statins with ezetimibe represents the logical first choice of therapy when LDL cholesterol is uncontrolled by statin monotherapy, with PCSK9 inhibitors considered for the highest risk categories on maximally tolerated statin plus ezetimibe therapies.
When the vote was returned to the audience, 2% said they would not change the treatment, 9% would use rosuvastatin 40 mg, 85% would add ezetimibe, 3% would add a PCSK9 inhibitor, and 2% would add a fibrate. The second vote showed that the vast majority of the audience agreed with the rationale given in the presentation to add ezetimibe and consider statin plus ezetimibe in secondary prevention of high-risk patients with T2DM.
Conclusion
In summary, the symposium’s take-home messages are that clinicians should move away from thinking about high-intensity statin therapy and instead consider high-intensity lipid- lowering therapy. Furthermore, patients that do not achieve the LDL cholesterol target with residual LDL cholesterol burden and high absolute risk require combination therapy, preferably with high-intensity statin and ezetimibe.