Meeting Summary
New guidelines for the diagnosis and management of chronic coronary syndromes (CCS)1were released during the 2019 European Society of Cardiology (ESC) Congress. The new guidelines were discussed in multiple sessions with different formats across the congress; this review summarises some of the discussion among experts at the congress around what the new guidelines mean for the way they manage their patients with respect to antithrombotic treatment.
A significant change in the new guidelines versus previous guidelines published in 20132is an update in nomenclature from ‘stable’ coronary artery disease (CAD) to chronic coronary syndromes, to reflect the fact that patients with CCS are at continuous risk of heart attacks, strokes, and death. This highlights the need for effective preventive therapy to protect against these thrombotic events and maintain a state of relative stability in patients with CCS. To this end, a new recommendation in the 2019 guidelines is to consider intensification of antithrombotic therapy, using aspirin plus another antithrombotic agent, to provide enhanced long-term protection for patients with CCS at high risk of ischaemic events. This review places the new guideline recommendations in clinical perspective, including thorough presentations of case studies to illustrate how patients at greatest risk of ischaemic events can be identified, and treatment stratified accordingly. These case studies highlight the role of dual pathway inhibition (DPI) in managing CCS patients with the greatest need for cardiovascular protection, who are likely to derive the greatest benefit from this treatment strategy.
Vascular Protection: When Do Patients Need More? Guideline Update
Professor Keith Fox
Prof Fox began the session by highlighting key issues in CCS and discussed how the new guidelines address these issues. Firstly, the previous standard of care was inadequate. Secondly, the concept of stable CAD is outdated: it is now recognised that there is a spectrum of risk in CCS, and a long-term risk of recurrent events persists even in periods of relative stability.
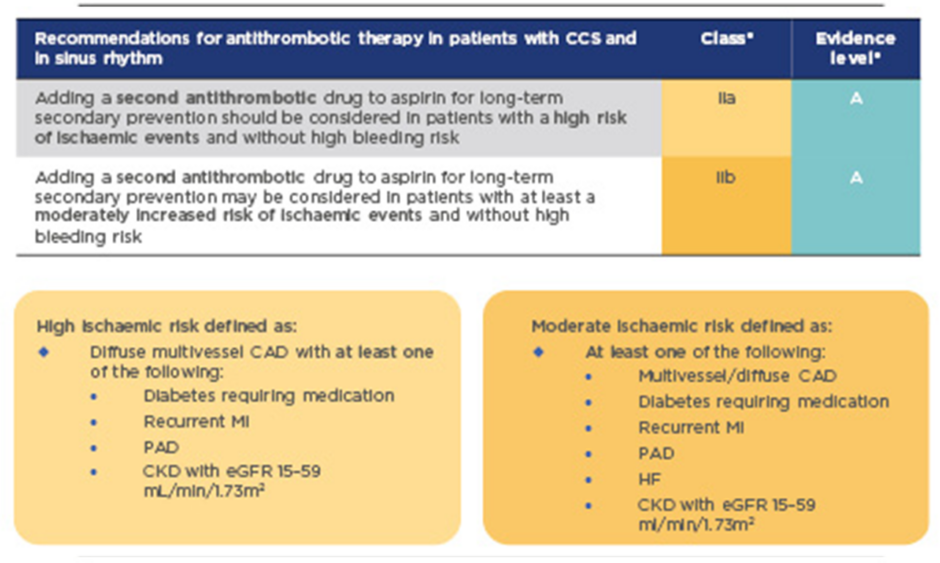
In the past, standard long-term treatment for stable CAD was aspirin monotherapy. New guidelines for CCS recommend considering adding a second antithrombotic agent to aspirin for long-term secondary prevention, for patients without high bleeding risk who are at high or moderate risk of further ischaemic events (Figure 1).1This is a Class IIa recommendation that should be considered for high-risk patients, and may also be considered for moderate-risk patients (Class IIb recommendation). High-risk patients are defined as those with diffuse multivessel CAD with at least one additional risk factor (diabetes requiring medication, recurrent myocardial infarction [MI], peripheral artery disease [PAD], or chronic kidney disease with estimated glomerular filtration rate [eGFR] 15–59 mL/min/1.73m2); moderate risk is defined as multivessel/diffuse CAD and/or at least one other risk factor from the above list, or heart failure (HF) (Figure 1). These risk factors have been identified based largely on a risk stratification analysis of the COMPASS study population, which identified high-risk groups as patients with ≥2 vascular beds affected, HF, renal insufficiency, or diabetes, and provided clear evidence for heightened benefits of a dual treatment strategy in these patient groups (Figure 2).3
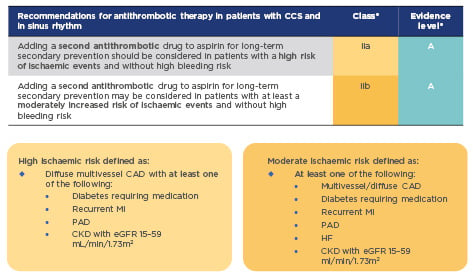
Figure 1: 2019 guidelines for the management of CCS: Recommendations for event prevention1
*A Class IIa recommendation is given if there is a divergence of opinion about the usefulness/efficacy of the given treatment but the weight of evidence/opinion is in favour of usefulness/efficacy; a Class IIb recommendation is given if usefulness/efficacy is less well established by evidence/opinion. Level of evidence A reflects data derived from multiple randomised clinical trials or meta-analyses.
CAD: coronary artery disease; CCS: chronic coronary syndromes; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HF: heart failure; MI: myocardial infarction; PAD: peripheral artery disease.
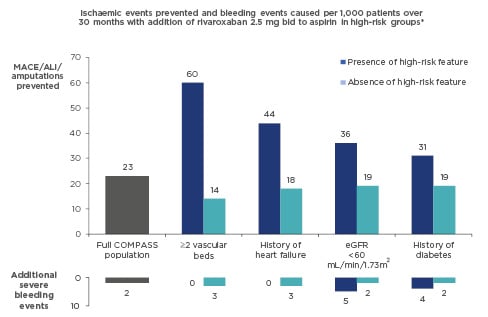
Figure 2: Identifying high-benefit patients for dual pathway inhibition: Risk-stratification analysis of COMPASS data3
*Identified through two independent methods (a modified REACH score and a CART analysis).
ALI: acute limb ischaemia; bid: twice daily; CART: classification and regression tree; eGFR: estimated glomerular filtration rate; MACE: major adverse cardiac events; REACH: REduction of Atherothrombosis for Continued Health.
The ESC guidelines present four antithrombotic agents as possible partners to use in combination with aspirin: clopidogrel, prasugrel, ticagrelor, and rivaroxaban (Table 1).1The first three are antiplatelet agents, acting via inhibition of the P2Y12receptor; their combination with aspirin constitutes dual antiplatelet therapy (DAPT). Rivaroxaban targets a different pathway, acting as an anticoagulant via inhibition of Factor Xa, and therefore provides dual pathway inhibition (DPI) when combined with the antiplatelet activity of aspirin. It is difficult to select a preferred treatment option in the absence of head-to-head trials (the guidelines simply list the treatments in alphabetical order), and individual factors make different treatments more suitable for different patients (discussed further later in this report). However, while results from separate studies with different agents cannot be directly compared, Prof Fox considered how convincing the evidence is for each option. Clopidogrel plus aspirin was investigated in the CHARISMA study, which was overall a negative trial (no significant effect on major adverse cardiovascular events, or cardiovascular or all-cause mortality), although there was a marginal benefit in a subgroup of patients with documented cardiovascular disease (as opposed to asymptomatic patients with atherosclerotic risk factors).4Prasugrel has some evidence for benefits in the first year of treatment (including new data presented at ESC 20195) but long-term evidence is lacking. Ticagrelor showed evidence of improved outcomes in the long-term treatment setting (1–3 years post-MI) in PEGASUS;6however, there was no significant impact on overall mortality, and a considerable increase in risk of major bleeding. The COMPASS study (the pivotal trial demonstrating efficacy of rivaroxaban 2.5 mg twice daily [bid] plus aspirin in patients with CAD and/or PAD)7provides the most convincing evidence of improvements in cardiovascular outcomes and overall mortality in this setting; indeed, the effect was so marked that the trial was terminated early, after an interim analysis revealed an excess of events in the aspirin-only arm compared with the rivaroxaban plus aspirin arm.

Table 1: Treatment options for dual antithrombotic therapy in combination with aspirin 75–100 mg once daily*1
*In patients with high or moderate risk of ischaemic events who do not have high bleeding risk
†5 mg od if body weight <60kg or age >75 years
‡Rivaroxaban is the only option for dual antithrombotic therapy indicated in patients with CCS at high ischaemic risk with or without a prior MI
bid: twice daily; CAD: coronary artery disease; CrCl: creatinine clearance; DAPT: dual anti-platelet therapy; MI: myocardial infarction; od: once daily; PCI: percutaneous coronary intervention.
All trials of dual antithrombotic therapy showed an increased risk of bleeding compared with monotherapy. The guideline recommendation for addition of a second antithrombotic agent therefore applies to patients without a high underlying risk of bleeding (defined as a history of intracerebral haemorrhage; ischaemic stroke, or other intracranial pathology; recent gastrointestinal [GI] bleeding; anaemia due to possible GI blood loss, or other GI pathology associated with increased bleeding risk; renal failure requiring dialysis or eGFR <15 mL/min/1.73m2; liver failure; bleeding diathesis or coagulopathy; or extreme old age or frailty). For all patients, it is necessary to weigh up potential treatment benefits versus bleeding risk. However, while ischaemic risk increases with accumulation of risk factors, bleeding risk increases at a much slower rate (based on analysis of data from the REACH registry)8leading to a more favourable benefit–risk balance for patients with high ischaemic risk.
Prof Fox closed the session by summarising key take-home messages: for patients with no elevated bleeding risk, there are now several options for long-term management. DAPT is well established in the setting of short-term treatment following acute coronary syndromes (ACS); guidelines now advocate extending this to long-term management of CCS for high-risk patients. DPI, adding an anticoagulant instead of a second antiplatelet agent, represents a new treatment paradigm, with compelling data from the COMPASS trial to support this approach. Speaking in a separate interview, Prof Jan Steffel, University Heart Center, Zurich, Switzerland, noted that although the COMPASS data are recognised by the cardiology community, formalising clinical practice recommendations based on those data in these guidelines should boost awareness of the need for ‘aggressive’ diagnosis and treatment for high-risk patients to reduce morbidity and mortality.
Guidelines in Perspective
Professor John Eikelboom
One challenge in CCS is the number of therapies that may be required to address all aspects of cardiovascular risk. Lifestyle modification is the foundation on which to build secondary prevention through medication, including treatments to lower lipids, and control blood pressure. Antithrombotic medication is an important part of the overall picture. Historically, aspirin has been the mainstay of antithrombotic treatment. Now, guidelines recommend considering intensification of antithrombotic therapy for patients at high risk of ischaemic events by adding a second antithrombotic agent for long-term secondary prevention.1
The new ESC guidelines do not distinguish between DAPT and DPI in the approach to intensifying antithrombotic treatment. Prof Eikelboom gave his perspective on the selection of a second antithrombotic agent. DAPT has been tested primarily in the post MI setting and is standard treatment for the first year following MI, and the first few months to 1 year following percutaneous coronary intervention (PCI). At that point, the need for intensified antiplatelet treatment should be re-evaluated. If the patient is no longer considered to be at high ischaemic risk, they can revert to single antiplatelet therapy (SAPT). If ischaemic risk remains high, the preferred course of action depends on what is driving that risk. If the primary concern is stent-related risk (e.g., multiple stents, long stent, or bifurcation stent), continued DAPT may be appropriate. However, if the main driver of ischaemic risk is atherosclerotic disease, then a dual pathway approach (using rivaroxaban in combination with aspirin) is likely to be more appropriate.
Prof Eikelboom noted that rivaroxaban 2.5 mg bid also reduced stent thrombosis in patients with ACS in the ATLAS study;9so, the DPI approach should not be ruled out in patients with stent-related risk, particularly if they also have additional risk factors relating to atherosclerotic risk. Rivaroxaban has the broadest indication of all options for dual antithrombotic therapy listed in the new ESC guidelines, as it’s the only option for which the indication is not restricted to the post-MI setting (Table 1). The guidelines support its use in patients who have been shown to derive the greatest benefit from the DPI approach, as identified in a risk stratification analysis of patients in the COMPASS study.3Indeed, the definition for high-risk patients provided in the new guidelines (described above in the Guideline Updatesection) was based largely on this analysis. Therefore, while the guidelines do not distinguish between DPI and DAPT in the recommendations for dual antithrombotic therapy, much of the direct evidence supporting dual antithrombotic therapy in high-risk patients is for DPI with rivaroxaban plus aspirin.
A query arose as to whether it was worth considering adjusting treatment, in light of the new guidelines, for patients who had been stable on SAPT for a long period. Prof Eikelboom confirmed that he would consider adding rivaroxaban for CCS patients with underlying atherosclerotic disease, even if they had been stable for many years. Speaking in a separate interview, Prof Jan Steffel noted that CCS patients are stable only in relative, but not absolute, terms, as reflected by the change in nomenclature in the new guidelines. Prof Martin Cowie, Imperial College London, London, UK, (interviewed separately) also highlighted this as an important aspect of the guidelines update, stating that there is “no such thing as stableCAD,” and emphasising the importance of assessing risk at each interaction with the patient to guide consideration as to whether they require amplified antithrombotic therapy.
FROM TRIAL TO TREATMENT IN TREATMENT IN VASCULAR PROTECTION: WHICH HIGH-RISK CORONARY ARTERY DISEASE PATIENTS BENEFIT THE MOST?
Case studies on clinical implementation of dual pathway inhibition in light of current guidelines.
Diabetes
Professor Gilles Montalescot
Prof Montalescot described the case of a 70-year-old woman with a long-standing history of hypertension and diabetes, managed using angiotensin converting enzyme (ACE) inhibitors and metformin. She had undergone PCI following MI, after which she received DAPT with aspirin plus ticagrelor, as well as lipid-lowering treatment with a statin. She was seen as an outpatient 13 months post PCI and was doing well. Her ECG was normal, and low-density lipoprotein controlled to 0.62 g/L, although there was some evidence of renal dysfunction, with creatinine clearance 54 mL/min. This appeared to be a relatively straightforward case, but Prof Montalescot addressed particular considerations in light of the new guidelines. He presented five options for continued management of this patient:
a) Stop ticagrelor (moving to aspirin monotherapy would have been the standard approach under the old guidelines for stable CAD).
b) Stop aspirin (ticagrelor monotherapy).
c) Continue aspirin plus ticagrelor (a regimen of ticagrelor plus aspirin was investigated in patients with diabetes and CAD in the THEMIS study).10
d) Switch to SAPT with clopidogrel.
e) Replace ticagrelor with rivaroxaban 2.5 mg bid (switch to DPI).
The audience were split between options (c) and (e). Prof Montalescot outlined a possible clinical decision-making pathway taking several factors into consideration:
- Bleeding risk; if bleeding risk is high, monotherapy would be more appropriate than combined therapy.
- Ischaemic risk.
i) Stent-related risk; elevated risk of stent thrombosis (which may be present if the patient has, for example, multiple stents, long stents, small-diameter stents in a small artery, or a prior history of stent thrombosis) may indicate continuation of DAPT.
ii) Clinical risk; several clinical risk factors, including polyvascular disease (≥2 diseased vascular beds), HF, diabetes, and renal dysfunction, place patients at increased risk of an ischaemic event. COMPASS data show a substantial benefit of DPI with rivaroxaban plus aspirin in these patient groups.
iii) For patients with low ischaemic risk, dual antithrombotic therapy may not be necessary, and the guideline recommendation to use a second antithrombotic agent in addition to aspirin does not apply to this group.
The patient featured in the case study did not have high bleeding risk or high stent-related risk but did have three identified clinical risk factors: age >65 years, diabetes, and renal dysfunction. According to an analysis of cumulative risk using REACH registry data for a subset of patients with ≥1 risk factor (consistent with enrolment criteria for COMPASS), a patient with three such risk factors would have approximately 17% increase in risk of an event over 4 years.8Therefore, Prof Montalescot considered her a suitable candidate for DPI with rivaroxaban plus aspirin. This is further supported by the COMPASS risk-stratification analysis, which showed that either diabetes or renal function alone would confer a greater benefit of the rivaroxaban plus aspirin regimen (compared with the average reduction in events seen in the overall study population);3combined, these risk factors suggest the patient stands to gain considerable benefit from this treatment strategy.
It was noted that many factors drive cardiovascular risk, and it is important to consider all sources of risk, including optimal control of blood pressure and lipids. The patient described in the case study was well controlled on ACE inhibitors and statins, but that is not always the case for many patients presenting in the clinic. It was suggested that she might benefit from a switch from metformin to a sodium-glucose co-transporter 2 (SGLT2) inhibitor to optimise glycaemic control. She did not have documented PAD; however, PAD is common in patients with diabetes, and the ESC recently published updated guidelines for diabetes11which recommend rivaroxaban (2.5 mg bid) plus low-dose aspirin for diabetes patients with lower extremity arterial disease.
Polyvascular Disease (Coronary Artery Disease/Peripheral Artery Disease)
Professor Dirk Sibbing
Prof Sibbing presented a case study featuring a 58-year-old male with multiple cardiovascular risk factors, including hyperlipidaemia, hypertension, and continued smoking. The patient had diffuse multivessel CAD and had undergone a coronary artery bypass graft several years previously, and multiple PCI in the intervening years, with a history of stent thrombosis and restenosis. He also had PAD affecting the bifurcations of the femoral arteries and carotid arteries bilaterally. He presented to the clinic with chest pain (angina pectoris) on exertion; angiography confirmed progression of the patient’s CAD on his existing SAPT treatment regimen (aspirin only), with stenosis requiring a further PCI (the patient’s sixth such procedure). He was prescribed atorvastatin and candesartan to manage his lipids and blood pressure. Prof Sibbing outlined options for antithrombotic treatment: initial treatment following PCI would be 6 months’ DAPT (aspirin plus clopidogrel or ticagrelor); options for long-term treatment included SAPT (revert to aspirin only), continued DAPT (e.g., aspirin plus ticagrelor), or DPI with aspirin plus rivaroxaban. This patient could have been considered a candidate for continued DAPT, given his history of stent thrombosis and restenosis (as discussed by Prof Eikelboom and Prof Montalescot); however, Prof Sibbing noted that CAD patients who also have PAD are at substantially increased atherosclerotic risk versus those with CAD only,12so this was a prominent concern. He revealed that he had chosen to treat the patient with aspirin plus rivaroxaban, switching to DPI for long-term secondary prevention. This treatment decision was taken prior to the recent release of the new ECS guidelines for CCS, and was based on convincing data from the COMPASS study demonstrating an enhanced benefit of aspirin plus rivaroxaban in patients with CAD+PAD, greater than that seen in patients with CAD only.13Risk of bleeding was similar in both patient subgroups, indicating a particularly favourable benefit–risk balance for patients with CAD+PAD.
Patients with polyvascular disease were among several subgroups identified in the COMPASS study population as being high-risk patients for whom DPI provided enhanced protection.3These data are now reflected in the new ESC guidelines for CCS,1supporting a Class IIa recommendation for addition of a second antithrombotic agent such as rivaroxaban for the long-term treatment of patients with high ischaemic risk.
Heart Failure
Professor Gilles Montalescot
Prof Montalescot’s second case study featured a 68-year old patient, a heavy smoker, who had undergone PCI following MI, and presented 16 months later with signs of HF. The patient improved on furosemide; he also remained on aspirin and prasugrel (initiated following PCI), as well as statins, ACE inhibitors, and beta-blockers.
Prof Montalescot considered options for continued management of this patient (based on the factors outlined when discussing his diabetes case study, above). The patient did not have an elevated risk of bleeding, so dual antithrombotic was not contraindicated. A patient with HF meets the criteria for moderate ischaemic risk according to the guideline’s definition; other risk factors such as smoking and age >65 years add to the patient’s overall risk profile.8Therefore, consideration of dual antithrombotic therapy was warranted. The patient was already on DAPT (aspirin plus prasugrel), so it was queried whether there was any need to change their treatment regimen. Prof Montalescot discussed circumstances in which a cardiologist might consider switching away from DAPT, namely when there are other risk factors besides stent-related risk to consider. The risk-stratification analysis from the COMPASS trial identified patients with polyvascular disease, HF, renal insufficiency, and diabetes as groups that derived the greatest benefit from DPI with aspirin plus rivaroxaban (Figure 2).3This provides an evidence-based rationale for considering a switch to DPI (replacing prasugrel with rivaroxaban) for this patient with HF.
Conclusion
Together, these case studies demonstrate several scenarios in which the availability of a new treatment option, DPI with rivaroxaban plus aspirin, can improve the management of patients with CCS who require additional cardiovascular protection. This is now supported by the new guidelines published by ESC.