Meeting Summary
Some individuals with diabetes, obesity, or metabolic syndromes are at risk of atherosclerotic cardiovascular (CV) disease (CVD) despite cholesterol-lowering therapies and/or statins.1,2 Omega-3 fatty acids, specifically eicosapentaenoic acid (EPA), at high doses have been associated with reductions in the risk of CV events by complex and multifactorial effects. Several investigations have shown that EPA, an omega-3 polyunsaturated fatty acid, reduces acute and chronic vascular inflammation and the development of atherosclerosis.3 These mechanisms are thought to contribute to atheroprotective effects that decrease the risk of atherosclerotic CVD events. Furthermore, if EPA was given to individuals with similar characteristics to the landmark trial populations, many CV events could be avoided.
Eicosapentaenoic Acid Ameliorates Vascular Inflammation: A Mechanistic Rationale for its Atheroprotective Effects
Doctor Anthony David Pisaniello and Doctor Stephen J. Nicholls
Atherosclerosis is an inflammatory disease and inflammation is crucial to all stages of atherogenesis. Targeting inflammation has the potential to reduce atherogenesis. Omega-3 fatty acids have been shown to reduce inflammation via several mechanisms, including peroxisome proliferator-activated receptor transcription pathways, inflammasome, and NF-κB, all of which are known to impact atherogenesis. However, the mechanisms involved in the atheroprotective effects of omega-3 have not been fully elucidated. This analysis of three studies explored whether omega-3 fatty acids can impact acute vascular inflammation, chronic vascular inflammation, and the development of atherosclerosis. The investigation also sought to determine if EPA and docosahexaenoic acid (DHA) had differential effects on these processes.
Study 1 was a double-blind, randomised, controlled trial and cell culture study of 40 healthy volunteers with low omega-3 intake at baseline. Participants were supplemented with 4 g daily of either EPA, DHA, fish oil with a 2:1 EPA:DHA ratio, or placebo for 30 days. The majority of the patients were female (70%) with a mean age of 38.5 years. All other baseline demographics were similar. Serum samples taken before and after supplementation were added to TNF-stimulated human umbilical vein endothelial cells in cell culture. Gene expression adhesion molecules and cytokine markers that are known to be important in atherosclerosis, including vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), and NF-кBp65, were measured by reverse-transcription PCR. Blood EPA and DHA levels were increased in all participants who completed the study. No significant differences were reported in resting heart rate, systolic blood pressure, high-sensitivity C-reactive protein levels, or cholesterol levels between the treatment groups. DHA was found to significantly reduce triglycerides by 27% (p<0.05) compared with all other treatment groups. EPA reduced the gene expression of MCP-1 by 25% (p<0.05) versus placebo. No other significant differences were found in the gene expression of the other markers in the EPA group versus the other treatment or placebo groups. Study 1 concluded that high-dose EPA supplementation delivered to endothelial cells in culture reduced the TNF-induced acute vascular inflammation and had superior effects compared with both DHA and standard fish oil.
Study 2 evaluated the effects of 30-day oral supplementation of 600 mg/kg/day of EPA, DHA, olive oil, or no treatment, via randomised allocation, on the protein expression of markers of acute vascular inflammation in 40 8-week-old, chow-fed, C57Bl/6 mice. A nonocclusive silastic collar model was used and fitted to the right common carotid artery. The collar produced an intense inflammatory response through the vessel wall. After 48 hours, the mice were humanely killed. Terminal blood samples were taken. Both carotid arteries were removed for immunohistochemical staining for VCAM-1, ICAM-1, MCP-1, and leucocyte marker CD18. All of the mice had normal post-treatment cholesterol and triglyceride levels, and there were no differences between the treatment groups. Collaring of the mice significantly induced protein expression of VCAM-1 (p<0.0001), ICAM-1, MCP-1, and CD18 (p<0.001 in all cases). EPA reduced VCAM-1 and MCP-1 expression by 43% and 41%, respectively, compared with the group receiving no treatment (p<0.005). High EPA and DHA levels correlated with lower expression of all markers, but a stronger correlation was found with EPA when the EPA:DHA ratio was analysed. Study 2 showed that EPA, but not DHA, reduced the protein expression of markers of acute vascular inflammation in a manner that was independent of cholesterol and triglycerides.
Study 3 assessed the impact of oral supplementation of 600 mg/kg/day of EPA, DHA, olive oil, or no treatment, via randomised allocation, on atherosclerotic plaque and lipid formation and chronic vascular inflammation using a diet-induced atherogenesis model in 40 8-week-old, apolipoprotein E-deficient mice. The mice received a 16-week atherogenic diet with supplementation for the final 8 weeks. At Week 8, a blood sample was taken and at Week 16 the mice were humanely killed. A total of 38 mice completed the study. At Week 8, all mice had high cholesterol levels (>500 mg/dL). Levels of total cholesterol increased in the last 8 weeks of feeding in the untreated (p<0.01) and olive oil groups (p<0.05), compared with the other groups. Total cholesterol levels stabilised in the EPA and DHA groups. EPA and DHA significantly reduced triglycerides by 29% (p<0.05) and 42% (p<0.001), respectively. One-half of the mice had total plaque burden, plaque collagen content, plaque macrophage content, and plaque smooth muscle cell content assessed; no significant differences were found between the treatment groups. No significant differences were reported when the aortic lipid burden was evaluated. EPA significantly reduced the gene expression of two markers of vascular inflammation (IL-1β and TNF-α; p<0.05), compared with the group without treatment. Furthermore, blood EPA concentrations correlated inversely with the gene expression of these markers (IL-1β: p=0.009, r=-0.63; TNF-α: p=0.04, r=-0.5). DHA did not significantly reduce the gene expression of these markers.
Taken together, these studies show that high-dose EPA reduces vascular inflammation throughout all stages of atherogenesis. Further studies are required to determine the mechanisms associated with these benefits. These data complement the findings and provide some evidence for the atheroprotective effects of EPA demonstrated in other clinical trials.
Elevated Triglycerides and Atherosclerotic Cardiovascular Disease Risk Reduction: It is Eicosapentaenoic Acid That Matters
Doctor Christie M. Ballantyne
Dr Ballantyne reviewed data from the JELIS4 and the REDUCE-IT trials,5 and provided an update on the status of the STRENGTH trial.3,6
The JELIS trial was a prospective, randomised, open-label trial that enrolled 18,645 Japanese males aged 40−75 years and females aged up to 75 years, with or without coronary artery disease (CAD) and with total cholesterol ≥250 mg/dL.4 Participants received either 1,800 mg of EPA daily with a statin (EPA group: n=9,326) or only a statin (control group: n=9,319). The primary endpoint was any major coronary event, including sudden cardiac death, fatal and nonfatal myocardial infarction (MI), and other nonfatal events including unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting. At the mean follow-up of 4.6 years, a 19.0% reduction in major coronary events was found; a major coronary event occurred in 2.8% of participants in the EPA group and 3.5% of participants in the control group (p=0.011; hazard ratio [HR]: 0.81; 95% confidence interval [CI]: 0.69−0.95).4 Post-treatment changes in low-density lipoprotein (LDL) cholesterol concentrations from baseline were similar in both groups: approximately 25%. A significant change from baseline in triglycerides was reported in the EPA group (p<0.001).4 The JELIS trial was the first prospective, randomised clinical trial to demonstrate prevention of coronary events by EPA.4 The reduction of CV events in JELIS were thought to be related to the on-treatment EPA levels and not the reduction in lipids.4
A subsequent analysis of the JELIS population assessed incident CAD, the number of CAD risk factors (hypercholesterolaemia, obesity, high triglyceride or low high-density lipoprotein [HDL] cholesterol, diabetes, and hypertension), and EPA treatment.7 Patients with hypercholesterolaemia who were receiving statin therapy, but did not have evidence of CAD (n=14,981), were randomly assigned to an EPA group (n=7,503) or a control group (n=7,478). In the high-risk subgroup, where patients had high triglycerides of >150 mg/dL and HDL cholesterol <40 mg/dL, EPA treatment reduced the risk of CAD by 53% (HR: 0.47; 95% CI: 0.23−0.98; p=0.043).7
A further subanalysis of JELIS aimed to evaluate the relationship between various plasma fatty acid concentrations and the risk of coronary events.8 In 15,534 participants, the HR was determined for major coronary events (sudden cardiac death, fatal or nonfatal MI, unstable angina pectoris, angioplasty or stenting, and coronary artery bypass grafting) relative to the on-treatment average level of plasma fatty acids. A higher plasma level of EPA (≥150 µg/mL; HR: 0.8) was associated with a reduced risk of major coronary events.8
REDUCE-IT was a landmark, Phase IIIb, randomised, double-blind, placebo-controlled trial that assessed if 4 g/day of icosapent ethyl, a highly purified ethyl ester of EPA, could reduce ischaemic events in 8,179 statin-treated patients with high triglycerides who were at an elevated CV risk.5,9 The REDUCE-IT patient population had established atherosclerosis or diabetes, plus ≥1 additional risk factor and persistent high triglyceride levels of 1.52–5.63 mmol/L (135–499 mg/dL) despite receiving statin therapy. The median follow-up was 4.9 years.5 The primary endpoint was incidence of five-point major adverse CV event (MACE), assessing time from randomisation to the first occurrence of CV death, nonfatal MI, nonfatal stroke, coronary revascularisation, or unstable angina requiring hospitalisation.5
The study found that participants in the icosapent ethyl arm (n=4,089) experienced a 24.8% relative risk reduction (HR: 0.75; 95% CI: 0.68−0.83; absolute risk reduction: 4.8%; number needed to treat: 21; p=0.00000001) in five-point MACE compared with participants in the placebo arm.5,10 The key secondary endpoint of CV death, MI, or stroke was also significantly reduced by 26.5% (HR: 0.74; 95% CI: 0.65–0.83; absolute risk reduction: 3.6%; number needed to treat: 28; p=0.0000006). Multiple subgroups were analysed, and consistent benefits were observed in both primary and key secondary endpoints for individuals receiving icosapent ethyl, including those with baseline triglycerides <1.69 mmol/L (<150 mg/dL).5
The JELIS design, interventions, and participant population were compared with that of the REDUCE-IT (Table 1). Dr Ballantyne highlighted the differences in the concentration of EPA used, the patient population, and the baseline EPA levels.4,5
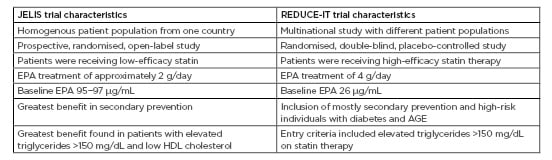
Table 1: JELIS trial and REDUCE-IT characteristics.
Adapted from Yokoyama et al.4 and Bhatt et al.5
AGE: advanced glycation end products; EPA: eicosapentaenoic acid; HDL: high-density lipoprotein.
The STRENGTH trial investigated omega-3 carboxylic acids (Epanova®, a mixture of DHA and EPA) versus corn oil in 13,000 participants with CVD, or at high risk for CVD with LDL cholesterol <100 mg/dL, triglycerides ≥200 to <500 mg/dL, and HDL cholesterol <40 mg/dL for males or <45 mg/dL for females, on statins.3,6 The endpoint was the first occurrence of MACE. The trial was discontinued in January 2020 due to lack of benefit. 3
In conclusion, the JELIS trial showed that treatments with EPA, not lipids, were associated with a reduction in CV events. The REDUCE-IT found that changes in triglycerides did not explain the high rates of CV risk reduction. Furthermore, EPA blood concentration was also thought to impact the degree of risk reduction observed in clinical trials.
Eicosapentaenoic Acid Levels and Cardiovascular Outcomes in the REDUCE-IT
Doctor Deepak L. Bhatt
As outlined above, the previously reported REDUCE-IT found significant reductions in the primary (p=0.00000001) and secondary endpoints (p=0.0000006) for individuals receiving icosapent ethyl compared with those receiving placebo.5 Using data from REDUCE-IT, subsequent analyses were performed that assessed the primary and key secondary composite endpoints by baseline serum EPA levels.
On-treatment serum EPA levels in the icosapent ethyl arm were assessed. The median percentage change in EPA levels from baseline was 393.5% at Year 1 (Year 1−5 range: 393.5–478.6%) which was sustained to Year 5 (p<0.0001). Over 5 years, the median change in serum EPA levels from baseline in the placebo arm ranged from -12.8% to 2.8%.
The analysis then tested the hypothesis that triglyceride reduction could contribute to CVD risk reduction.
This was explored by a stratified analysis of the time to the primary endpoint by adjusting time-varying covariates of post-baseline biomarkers.
The impact of the on-study changes in triglycerides (HR: 0.77; 95% CI: 0.70−0.85) on the overall primary composite endpoint (HR: 0.75; 95% CI: 0.68−0.83) contributed an approximately 2.0% reduction to the overall observed 24.8% risk reduction. A similar limited impact was found on other biomarkers including LDL cholesterol, HDL cholesterol, non-HDL cholesterol, apolipoprotein B, high-sensitivity C-reactive protein, and remnant-like particle cholesterol. Covariate analysis of on-treatment serum EPA levels found that they accounted for almost all of the 25% relative risk reduction in the primary endpoint, with an adjusted covariate HR of 1.03 (95% CI: 0.91−1.16). Therefore, serum EPA levels are highly correlated to atherosclerotic CVD risk reduction.
To explore this finding further, EPA levels as a continuous variable were analysed. High EPA levels were associated with significant decreases in the HR of the primary endpoint, the key secondary endpoint, CV death, and total mortality (p<0.001). These data suggest that the higher the serum EPA level, the greater the CV benefit observed. Similar correlations were also noted in fatal and nonfatal MI, fatal and nonfatal stroke, coronary revascularisation, and unstable angina requiring hospitalisation (p<0.001). Patients with either established CVD or diabetes with risk factors at baseline also demonstrated a dose-response reduction in the HR with increasing EPA levels (p<0.001). A consistent benefit was observed with increasing EPA levels for sudden cardiac death and cardiac arrest. In REDUCE-IT, new heart failure and new heart failure requiring hospitalisation did not achieve significant risk reductions overall. However, this analysis found that increasing serum EPA levels were significantly associated with risk reduction (p<0.001) for these endpoints. These data suggest that to reduce the risk of some CV outcomes, high EPA levels
are required.
The study had some limitations. Approximately 14% of patients did not have baseline EPA levels, but baseline characteristics were similar. Study outcomes between participants who did or did not have baseline EPA data were also similar. Data for on-treatment dose-response HR for bleeding, serious bleeding, or atrial fibrillation or flutter are not yet available. The findings from this analysis only apply to the specific formulation used. A lack of benefit observed with other omega-3 fatty acid studies may be due to the formulation used, as other mixtures contain both EPA and DHA which have differing biological effects.
Dr Bhatt concluded that compared with placebo, icosapent ethyl (4 g/day) significantly reduces first (p=0.00000001) and total CV events (p=0.0000000004),5 and the benefits are beyond those explained by the degree of triglyceride or other biomarker changes. On-treatment EPA levels correlate with multiple CV endpoints and underpin the data reported in the landmark REDUCE-IT.
REDUCE-IT Eligibility and Preventable Cardiovascular Events in the USA Population: An Analysis of the National Health and Nutrition Examination Survey
Doctor Nathan D. Wong
The impact of icosapent ethyl for the prevention of CV events in individuals who meet the REDUCE-IT eligibility criteria on a national level is unclear. An analysis was performed to estimate the number of preventable CVD events if adults in the USA who met REDUCE-IT eligibility criteria5 were given icosapent ethyl (4 g/day). The study identified suitable adults who met the REDUCE-IT inclusion criteria using data obtained from the National Health and Nutrition Examination Surveys (NHANES) 1999−2016. General inclusion criteria included triglycerides of 135−499 mg/dL, HbA1c <10%, blood pressure <200/100 mmHg, and treatment with a statin with LDL cholesterol of 40−99 mg/dL. Estimates were calculated for two prevention groups.
The primary prevention group had no history of CVD but had Type 1 or 2 diabetes mellitus requiring treatment, were aged ≥50 years, and had one of the risk factors for CVD (cigarette smoking or history of smoking within the past 3 months, hypertension, blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic, antihypertensive medication, HDL cholesterol ≤40 mg/dL for males or ≤50 mg/dL for females, or micro- or macroalbuminuria with albumin:creatinine ratio ≥2.5 mg/mmol).
The inclusion criteria for the secondary prevention group were self-reported and included a diagnosis of coronary heart zdisease, MI, or stroke.
Data regarding carotid or peripheral arterial diseases were not available. The analysis estimated primary (CVD death, nonfatal MI, stroke, revascularisation, or unstable angina) and secondary composite (CVD death, nonfatal MI, or stroke) events using overall REDUCE-IT initial and total published event rates and REDUCE-IT USA initial event rates in the icosapent ethyl and placebo groups. The difference between these groups was the number of preventable events.
From an initial sample of 11,445 adults aged >45 years representing 111.1 million individuals, a final sample meeting the REDUCE-IT eligibility criteria (both primary and secondary prevention groups) included 319 individuals that represented 3 million individuals. Of these, 64.2% (n=205; N=1,908,781) had experienced prior CVD, and 35.7% (n=114; N=1,133,110) had diabetes and were aged >50 years with at least one additional risk factor. The study compared the REDUCE-IT overall placebo group demographics with the NHANES eligible population and found that the NHANES population had fewer males (59.8% versus 70.8%), a higher proportion of individuals in the primary prevention cohort (37.3% versus 29.3%), and lower median triglyceride levels (180.6 mg/dL versus 216.0 mg/dL). BMI >30 was slightly higher in the NHANES group compared with the REDUCE-IT placebo group (61.6% versus 57.8%). The cohorts were similar in all other demographic characteristics, including age and diabetes prevalence.
The study estimated that by giving REDUCE-IT-eligible persons icosapent ethyl for 4.9 years, a total of 71,391 primary composite outcome events per year and 31,660 secondary composite outcome events per year could be prevented (Figure 1A). Of these events, 29,798 initial events per year and 22,349 initial events per year could be avoided for primary and secondary composite outcomes, respectively. In a subset analysis using the REDUCE-IT USA event rates, it was estimated that 40,351 primary composite outcome events and 27,936 secondary composite outcomes events each year could be avoided (Figure 1B). Overall, these data show that if statin-controlled American adults with known CVD or diabetes and additional risk factors received icosapent ethyl, a large number of CV events could be prevented.
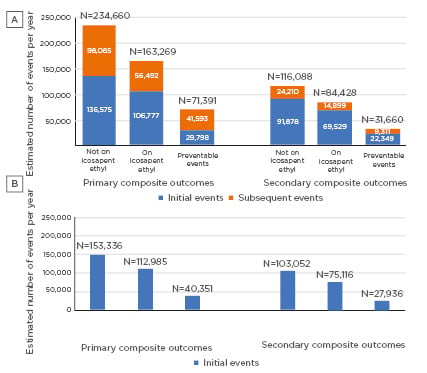
Figure 1: A) Distribution of annual projected initial and subsequent preventable primary and secondary composite endpoint events in the USA if eligible individuals were on icosapent ethyl (NHANES 1999−2016). B) Distribution of annual projected initial preventable primary and secondary composite endpoint events in the USA if eligible individuals were on icosapent ethyl based on REDUCE-IT USA results (NHANES 1999-2016).
NHANES: National Health and Nutrition Examination Survey.








