Co-chairs: Lars Lund,1 Ovidiu Chioncel2,3
Speakers: Ahmad Masri4
1. Karolinska Institutet, Stockholm, Sweden
2. Prof. Dr. C.C. Iliescu Institute for Emergency Cardiovascular Diseases, Bucharest, Romania
3. University of Medicine Carol Davila, Bucharest, Romania
4. Oregon Health & Science University Medical Group, Portland, USA
Disclosure: Lund is supported by the Karolinska Institutet, the Swedish Research Council (grant 523-2014-2336), the Swedish Heart Lung Foundation (grants 20150557 and 20190310), and the Stockholm County Council (grants 20170112 and 20190525); has received grants from AstraZeneca, Vifor, Boston Scientific, Boehringer Ingelheim, Novartis, and MSD; has received consulting fees from Vifor, AstraZeneca, Bayer, Pharmacosmos, MSD, MedScape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, Servier, Edwards Life Sciences, and Alleviant; has received speaker’s honoraria from Abbott, OrionPharma, MedScape, Radcliffe, AstraZeneca, Novartis, Boehringer Ingelheim, and Bayer; has a patent with AnaCardio; and has stock ownership in AnaCardio. Chioncel has declared no conflicts of interest. Masri has received consulting honoraria and royalties from Attralus, Bristol Myers Squibb, Cytokinetics, Eidos Therapeutics, Ionis Pharmaceuticals, and Tenaya Therapeutics; and has held research contracts with Akcea Therapeutics, Ionis Pharmaceuticals, Pfizer, and Ultromics.
Acknowledgements: Writing assistance was provided by Helen Boreham, Boston Spa, UK.
Support: The publication of this article was supported by Cytokinetics. The views and opinions expressed in this article belong solely to the named speaker.
Disclaimer: Aficamten is an investigational agent and is not approved for any indication by the U.S. Food & Drug Administration (FDA) or European Medicines Agency (EMA).
Citation: EMJ Cardiol. 2022;10[Suppl 5]:2-7. DOI/10.33590/emjcardiol/10153530. https://doi.org/10.33590/emjcardiol/10153530.
Presentation Summary
This presentation was part of the ‘Registries and Trial Updates II’ session of the European Society of Cardiology’s (ESC) Heart Failure 2022 Congress on 23rd May 2022, held in Madrid, Spain. Ahmad Masri, Medical Doctor and Director of the Hypertrophic Cardiomyopathy Centre at Oregon Health & Science University, Portland, USA, presented interim results from the ongoing REDWOOD-HCM open-label extension (OLE) study, which is evaluating the efficacy and safety of aficamten, a next-in-class cardiac myosin inhibitor, in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM). In this OLE study, aficamten demonstrated sustained efficacy, similar to the parent trial, achieving significant reductions in left ventricular outflow tract (LVOT) gradient, improvement in heart failure symptoms, and decreases in N-terminal pro B-type natriuretic peptide (NT-proBNP). Aficamten also proved well tolerated in patients with obstructive HCM, with no events of left ventricular ejection fraction (LVEF) <50% attributable to study drug, and no permanent discontinuations. The treatment effect of aficamten was durable for up to 6 months in the OLE study.
Interim Results From the REDWOOD-HCM Open-Label Extension Study of Aficamten
Aficamten is an investigational, next-in-class, small molecule, allosteric, and selective cardiac myosin inhibitor that exerts its therapeutic effects by decreasing myocardial contractility. It is currently undergoing clinical evaluation as an oral, once-daily drug therapy to treat heart failure symptoms and improve functional capacity in patients with symptomatic, obstructive HCM. By targeting the underlying mechanism of hypercontractility in HCM, aficamten aims to decrease or abolish LVOT gradients (LVOT-G), and substantially improve heart failure symptoms.
REDWOOD-HCM-OLE is an ongoing open-label extension study assessing the long-term safety and efficacy of aficamten (CK-3773274) in patients with obstructive HCM over a 5-year period. This study builds on the completed Phase II REDWOOD-HCM trial,1 in which aficamten proved well-tolerated and achieved decreases in LVOT gradients, improvements in New York Heart Association (NYHA) functional class, and reduction in key cardiac biomarkers. REDWOOD-HCM enrolled three cohorts of patients, all of whom were invited to screen and enroll in the OLE study. Cohorts 1 and 2 were placebo-controlled in design and included 21 and 20 patients, respectively, with obstructive HCM, who were treated with differing doses of aficamten ranging between 5–15 mg (Cohort 1) and 10–30 mg (Cohort 2). Cohort 3 was an open-label single arm design for patients (n=13) who remained obstructed on disopyramide and received add-on therapy with aficamten (between 5–15 mg).
REDWOOD-HCM Open-Label Extension Study Design and Participant Characteristics
During this oral presentation at the ESC’s Heart Failure 2022 Congress,2 Masri presented the study design details of the ongoing REDWOOD-HCM OLE trial (Figure 1). He explained that after screening and enrollment, patients entered a 6-week period of up-titration, where from Day 1, the dose of aficamten could be adjusted every fortnight to reach the “maximum desired effect.” Echo-guided dose titration based on the site reads was managed by the principal investigator who had the flexibility to change the dose titrated at any time during the trial. No clinical pharmacokinetic monitoring was conducted as part of this study. After the echo-guided dose titration period, subsequent site visits occurred once every 3 months. There is also a cardiac MRI sub-study, which is being offered to all eligible participants, Masri added.
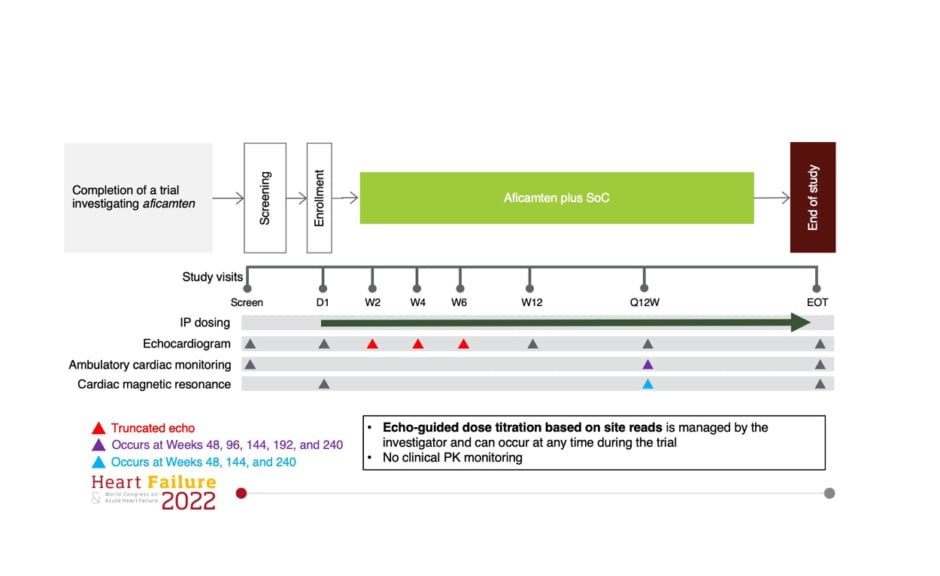
Figure 1: REDWOOD-HCM open-label extension study design.
D1: day 1 ; EOT: end of trial; IP: investigational product; PK: pharmacokinetic; Q12W: injection dosing regimen every 12 weeks; SoC: standard-of-care; W2: Week 2; W4: Week 4; W6: Week 6; W12: Week 12.
Masri presented the baseline characteristics of the 38 patients to date who have been enrolled into the REDWOOD-HCM OLE study. Patients’ mean age was approximately 60 years, and 57.9% were female. More than half of study participants had NYHA Class III (52.6%), with the remainder having NYHA Class II (47.4%). Masri commented that “patients were well treated on background medical therapy,” with over one-quarter on disopyramide (26.3%). A further 78.9% and 28.9% of study participants were receiving background treatment with β-blockers and calcium channel blockers, respectively. Patients in the REDWOOD-HCM OLE also displayed elevated cardiac biomarkers at baseline; mean NT-proBNP was 628.1 pg/mL, and mean cardiac troponin I was 13.8 ng/L. The overall mean duration on treatment was around 26 weeks.
Aficamten dosing over time in the REDWOOD-HCM OLE study was driven by site-read echo-guided dose titration criteria (Weeks 2, 4, 6, 12, and 26). All patients initiated treatment with 5 mg aficamten on Day 1. Patients were then up-titrated if they had an LVEF of 50% or above, yet remained obstructive, defined as a resting LVOT-G ≥30 mmHg or Valsalva LVOT-G ≥50 mmHg. Conversely, patients were down-titrated if their LVEF was less than 50%. Aficamten dose interruptions were implemented if patients’ LVEF dropped below 40%. Masri explained that, by Week 12 and Week 24 of the study, the majority of patients were receiving either a 10 or 15 mg dose of aficamten, with only a small minority remaining on the original 5 mg starting dose.
REDWOOD-HCM Open-Label Extension Interim Results
Masri went on to showcase key interim results from the REDWOOD-HCM OLE, highlighting how aficamten achieved a rapid and sustained reduction in resting LVOT gradient (Figure 2). Regarding resting LVOT gradient, Masri noted: “As early as 2 weeks, you see a rapid reduction in resting gradients, which continues to improve and is sustained over time.” Aficamten demonstrated similar effects on Valsalva LVOT-G, producing an initial reduction at 2 weeks, which was followed by a continued decrease in the LVOT gradient over time throughout the study. Aficamten maintained resting and Valsalva LVOT-Gs below these key cut-off points from 2 and 4 weeks, respectively, and for the remainder of the study duration. Decreases were statistically significant (p<0.001) for both gradient measures from Week 2 onwards. Aficamten treatment also demonstrated a minimal and stable reduction in LVEF over 24 weeks, with significance from 4 weeks (p <0.05), confirming its “gentle effect on LVEF throughout the study,” commented Masri.
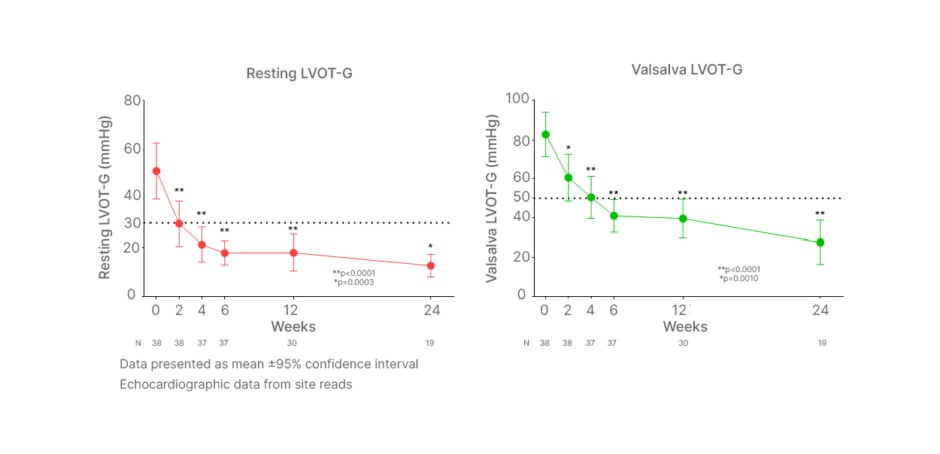
Figure 2: REDWOOD-HCM open-label extension rapid and sustained reduction in left ventricular outflow tract gradient with aficamten.
Dotted lines denote clinically significant 30 mmHg and 50 mmHg threshold levels for resting LVOT-G and Valsalva LVOT-G, respectively.
LVOT: left ventricular outflow tract; LVOT-G: left ventricular outflow tract gradient.
In addition to reductions in LVOT-Gs, aficamten demonstrated improvements in NYHA class over the course of the OLE study. At baseline, 53% of patients were in Class III and 47% in Class II, recapped Masri; however, by Week 12, “there were significant improvements,” with 41% of patients in Class I, 52% in Class II, and only 7% remaining in NYHA Class III (p<0.0001). These results were mirrored at Week 24, at which point 56% of patients had attained NYHA Class I, 39% were in Class II, and only 6% persisted in Class III. A different way of looking at these results is the proportion of improvement versus no change, explained Masri. Overall, around 80% of patients treated with aficamten showed improvement by one or two NYHA classes during the course of the study, while approximately 20% did not show any change. Most of those patients with no change were in Class II. No patients had a worsening of NHYA class from baseline during treatment with aficamten in the OLE.
Aficamten also produced significant decreases in key cardiac biomarkers from baseline over the course of the REDWOOD-HCM OLE study. Masri described how decreases in cardiac biomarkers of both wall stress and subclinical myocardial injury were observed during aficamten treatment, reflected by a 70% reduction in NT-proBNP (p<0.0001) and a 20% reduction in cardiac troponin I (p=0.002), respectively, compared with baseline.
Turning to safety findings from the REDWOOD-HCM OLE study, Masri explained that the tolerability profile of aficamten was favourable. Table 1 summarises the rate of treatment-emergent adverse events (TEAE) occurring during treatment with aficamten, “none of which were unexpected,” noted Masri. The adverse events (AE) that occurred in more than one patient in the study included headache (n=4), dizziness (n=3), alopecia (n=2), atrial fibrillation (AF; n=2), diarrhoea (n=2), fatigue (n=2), parosmia (n=2), rash (n=2), and sinusitis (n=2). Importantly, no permanent discontinuations of aficamten occurred due to TEAEs, with only one patient requiring a dose interruption as a result of TEAEs, and TEAEs leading to dose reductions in two patients.
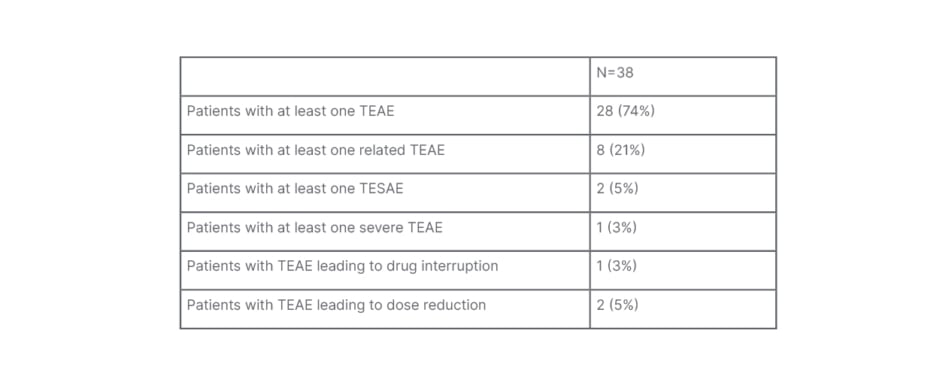
Table 1: Safety in the REDWOOD-HCM open-label extension study.
TEAE: treatment-emergent adverse event; TESAE: treatment-emergent serious adverse event.
In further discussion of the safety profile, Masri focused on three specific AEs from the OLE study, which he described as “not related to aficamten but important to explain.” The first case was a patient with LVEF <50%, who experienced a treatment-emergent serious AE while receiving aficamten therapy. This patient had a history of alcohol-related AF and, prior to the study before receiving any treatment with aficamten, their LVEF was consistently reduced in the range 45–50%. After recovering sinus rhythm, this patient entered the REDWOOD-HCM OLE trial and began treatment with aficamten. However, while receiving treatment with aficamten 15 mg, the patient experienced a recurrent episode of alcohol-induced AF with similar reduction of LVEF to 47%. At this point, the dose of aficamten was down-titrated. The patient went on to have a subsequent failed cardioversion, leading to interruption of aficamten treatment. As things currently stand, this patient has returned to sinus rhythm on amiodarone, is abstinent from alcohol, has an LVEF of 60% with evidence of obstruction, and has successfully restarted aficamten at a dose of 5 mg.
The second case described a patient who required temporary down-titration of aficamten. In this instance, during the site-read echo, the investigator became concerned about corrected QT interval (QTc) prolongation in a patient with an abnormal baseline electrocardiogram. Down-titration of the aficamten dose was therefore performed pending core-lab QTc interpretation. The core lab subsequently confirmed that the patient’s QTc was in fact normal, and the aficamten dose was then increased.
REDWOOD-HCM Open-Label Extension Adverse Events and Safety Data
The third and final case related to a severe treatment-emergent serious AE in a patient enrolled in the OLE study. This patient displayed altered mental status prior to planned cardioversion for AF on direct-acting oral anticoagulants, which led to their hospitalisation. An MRI was carried out, which showed that the patient had experienced a presumed embolic stroke. The patient was subsequently diagnosed with a congenital cardiac abnormality, in the form of a secundum atrial septal defect. No down-titration or interruption to the aficamten dose was required in this case, stressed Masri.
Masri concluded his presentation with a summary of the key take-home messages from the interim results of the REDWOOD-HCM OLE study. In this study, patients with obstructive HCM treated with background medical therapy including disopyramide, aficamten was associated with significant and sustained reductions in LVOT gradients and produced substantial improvement in heart failure symptoms, with around 80% of patients experiencing an improvement in at least one NYHA class. Treatment with aficamten was also associated with significant reductions in the key cardiac biomarkers, NT-proBNP, and high-sensitivity cardiac troponin I. Over the course of the OLE study, aficamten proved well tolerated, with no events of LVEF <50% attributed to the study drug. To date, there has been a single dosing interruption and no permanent discontinuations of aficamten therapy.
These data demonstrate that the treatment effect of aficamten is durable for up to 6 months, noted Masri. Moving forward, the 20 mg dose of aficamten is now available for use in the REDWOOD-HCM OLE trial for patients who may not have achieved target gradients on the 15 mg dose. Aficamten is also being evaluated in the ongoing SEQUOIA-HCM study, which is a pivotal Phase III trial in patients with obstructive HCM.
Post-presentation Question and Answer
A very brief window of opportunity for questions followed the presentation of the REDWOOD-HCM OLE interim data.
Session co-chair, Ovidiu Chioncel, Prof. Dr. C.C. Iliescu Institute for Emergency Cardiovascular Diseases, Bucharest, Romania, and University of Medicine Carol Davila, Bucharest, Romania, described the study as “very elegant”, demonstrating “a significant decrease in LVEF gradient and significant improvement in NYHA class” with aficamten. However, this being a “very small study”, Chioncel questioned whether any data were available on concordance; how many patients simultaneously improved in terms of gradient and functional class, and how many patients improved only in terms of gradient and how many improved only clinically?, he asked.
Masri acknowledged that he did not have the exact numbers; however, he responded that the majority, if not all, of patients treated with aficamten had achieved improvements in their gradients, and only 20% did not show improvement in NYHA class. “So it’s near universal that you see the improvement in gradients,” he commented. Masri continued that, for those patients who did not achieve the desired cutoffs for their gradients, this was because the dose of aficamten was capped at 15 mg. Now that the 20 mg dose is available, moving forward, “this will allow us to achieve even further reductions in gradients,” he concluded.
Conclusion
Overall, the compelling clinical results obtained to date from both the REDWOOD-HCM trial of aficamten and the ongoing OLE study support the continued investigation of this next-in-class cardiac myosin inhibitor as a novel treatment option for obstructive HCM. The pivotal Phase III study SEQUOIA-HCM is currently underway, investigating the clinical efficacy and safety of aficamten in a larger cohort of patients with obstructive HCM, and is due for completion in change to Q1 2024.








