Abstract
Heart disease affects much of the world’s population, yet many people have no idea that they could have something wrong with them. An opportunity therefore exists for targeted screening for conditions such as cardiovascular disease, heart rhythm changes, valvular heart disease, structural abnormalities, and more subtle, rarer inherited heart conditions. At the same time, the rapid development of digital health technologies and clinical support systems is providing patients and their doctors access to augmented intelligence solutions to diagnose these conditions. This article will focus on how the emerging field of digital health technology can aid screening for heart disease and explore its usefulness in disease specific and population specific groups.
Key Points
1. The use of digital health technologies, including mobile devices and artificial intelligence, in screening for heart disease is a developing field, where unanswered questions include which populations or conditions might benefit from screening, as well as the role for incidental screening.2. Digital devices can screen large population groups for multiple pathologies, and are able to provide data previously inaccessible, such as heart rhythm prior to first collapse.
3. Digital health is a rapidly expanding field with substantial ongoing developments; if these advances are evaluated properly, it is a field with great potential for screening populations for heart disease.
INCIDENCE AND PREVALENCE OF HEART DISEASE
Cardiovascular disease (CVD) is one of the leading causes of mortality and morbidity worldwide, and was the leading cause of death globally in 2019.1 This is despite continued improvement in its management and the reduction of coronary artery disease (CAD).2 One reason for this is the presence of undiscovered conditions in certain individuals. For instance, there are an estimated 260,000 people with familial hypercholesterolaemia in the UK, but only 6–7% of them are diagnosed. Furthermore, over 600 people under 35 years old die a year in the UK from an unrecognised heart condition.1,3 There are particularly prominent examples of younger victims with sudden cardiac death (SCD) in professional athletes. These draw the public’s attention and, whilst uncommon, are dramatic and have a high burden in terms of life-years lost.4
CVD also has significant economic costs, with an estimated 19 billion GBP per year impact on the UK’s economy and around 106 billion EUR across the European Union (EU).1,2 The National Institute for Health and Care Excellence (NICE) in the UK estimated that a 1% drop in cardiovascular risk would prevent 25,000 CVD cases, producing 40 million EUR a year in savings.5
SCREENING
The purpose of screening is to detect a disease in its early stages and treat it to reduce morbidity, mortality, and the associated societal and healthcare costs.6 In an increasingly digital age with many innovations, there are new opportunities to utilise technology to screen for pathology. History, physical examination, ECGs, echocardiograms, CT, and genetic screening can all be utilised. The usefulness of these methods varies depending on the condition being screened. This article will focus on how the emerging field of digital health technology affects screening for CVD and explore its utility in disease- and population-specific screening.
Disease-Specific Screening
Sudden cardiac death
There remains significant debate around the optimal methods of cardiac screening for SCD in the young. The European Society of Cardiology (ESC) recommends screening of high-risk individuals, including athletes, whereas the American Heart Association (AHA) guidelines recommend just screening athletes. There are other discrepancies; the ESC endorses the use of ECGs in screening, but the AHA do not.7,8 This is particularly interesting in the context of the leading causes of death in young competitive athletes. Additionally, a 12-lead ECG can increase the sensitivity of screening and there is good evidence supporting its cost-effectiveness.9,10 There are papers suggesting the focus on athletes ignores the damaging effects SCD in a non-athlete can have on friends, peers, and family; especially given the number of population-wide SCDs that happen during sleep, which could be as high as 40%, and those that occur in non-athletic groups.4,11,12
The risk of false positives and the harm this causes is often cited as the reason more generalised screening is not recommended.13,14 However, screening may also be cheaper than assumed and could be employed in younger age groups. Some papers suggest relying on improving resuscitation over screening is unsatisfactory due to SCD’s poor survival rate and the increased data gathered can be used to further refine screening.11,15 The technologies mentioned later in this article show promise for collecting such data at a reduced cost and raise the possibility of being able to recover data just preceding events, such as SCD. This is without taking into account the potential for artificial intelligence (AI) to revolutionise risk prediction or early warning systems.16
Atrial fibrillation
Targeted screening can also assist in the detection of atrial fibrillation (AF) due to its often insidious nature and the subsequent damaging thromboembolic event.17 The ESC recommends opportunistic screening for AF in anyone older than 65 years due to the risk of ischaemic stroke and increased mortality associated with asymptomatic AF.18 Several studies suggest the use of a manual pulse check with supplementation from single-lead or 12-lead ECG devices due to the ESC requirements for diagnosis.17-19
In the 2020 ESC guidance, the sensitivity and specificity of the various AF screening tools were compared with the gold standard of a 12-lead ECG (Table 1).18 There are two large studies, the Apple Heart study, with over 400,000 self-enrolled participants, and the Huawei Heart study, with 200,000. Both studies showed promise for the use of smartwatches and photoplethysmography (PPG) in screening for arrhythmia.18,20,21
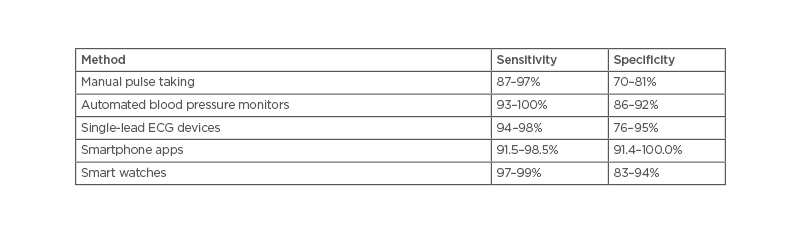
Table 1: The sensitivity and specificity of the various atrial fibrillation screening tools.18
A recent meta-analysis supported the systematic and opportunistic screening for AF.22 While it did not examine smartwatches or PPG, it did find that systematic, rather than opportunistic screening, was more effective at identifying patients with AF; however, it was noted that this may not be cost-effective. Others suggest this may be achieved by lowering the age cut off to 40 years old.22 This, combined with the Apple Heart and Huawei Heart studies not requiring in-person appointments, may increase cost-effectiveness further.20,21 The portable single-lead ECG devices have shown promise by increasing the ease and reducing the cost of screening,17,23,24 and could increase the potential for including wider populations.17,22
Coronary artery disease
CAD is one of the leading causes of death worldwide.25 Due to its potentially silent nature there has been great interest in screening, but the optimal approach is debated. Initial screening can involve history and examination to establish risk factors for CAD, alongside blood tests and scoring systems such as the Systemic Coronary Risk Estimation 2 (SCORE2) score.26 These take information such as blood pressure, lifestyle factors, family history, and sex into account alongside co-morbidities. The ESC recommends assessing risk in males over 40 years old and females over 50 years, unless there are known CVD risk factors. Most of the ESC recommendations discuss prevention using lifestyle and medications to adjust modifiable risk factors rather than exploring screening as an option. This makes it difficult to assess the ESC position on more generalised screening.26
In recent years, coronary artery calcium scoring and CT coronary angiography (CTCA) have come to the fore in assessing patients’ CAD risk in a non-invasive manner.27 There are also new ways to assess CAD risk using CTCA in combination with AI. One example is the CaRi-Heart® (Caristo, Oxford, UK), which uses images captured during a standard CTCA alongside traditional risk factors. By calculating the Fat Attenuation Index (FAI), which has been previously validated,28,29 in combination with traditional risk factors, it determines an 8-year absolute risk of a fatal cardiac event, or the CaRi-Heart risk. Inflammation has long been suspected as having a key role in CAD,30 but until FAI was established there was no straightforward way to measure this.28 Caristo takes FAI a step further and has been demonstrated to show a significant clinical benefit over traditional CVD risk factors and has the potential to enhance the utility of CTCA in the risk stratification of CAD.31
Fractional flow reserve also uses CTCA and AI-powered algorithms to establish vessel-specific ischaemia and flow obstruction. It is recommended in British32 and European27 guidance for the risk stratification of those with stable chest pain. Not least in part due to its prognostic value and potential to increase the accuracy of assessing risk in an individual as well as helping select who undergoes invasive strategies such as direct invasive coronary angiography.33
In December 2021, the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) published their recommendations for non-invasive imaging in coronary syndromes.34 This not only supported the use of fractional flow reserve, but also recommended the use of echocardiograms, especially stress echocardiography, which can also be combined with the power of AI. The EchoGo Pro (Ultromics Dallas, Texas, USA), for instance, uses AI to automatically analyse stress echocardiograms, which it can then use to risk stratify the likelihood of severe CAD in an individual.35 This was shown to be 10% more sensitive than a manual assessment.35
Valves
Due to increasing life expectancy, the prevalence of valvular heart disease (VHD) is rising. In Europe, approximately one million people over the age of 75 years suffer from severe aortic stenosis.36 A study in 2016 screened for VHD in primary care patients over the age of 64 years and discovered just over half had previously undetected VHD. Fortunately, the majority had mild disease; however, 6.4% had clinically significant disease.37 They also established a strong association with AF, which suggests this could be a targeted area for screening. Currently, there are no population screening programmes for VHD in adults.
Population-Specific Screening
For screening to be relevant to an individual, one must consider the disease(s) to screen for. When screening certain populations, cardiologists can screen for many cardiac diseases with the same test, being mindful that there will be different incidences in different populations.
Athlete screening
There are differences in the recommendations and methods of pre-participation cardiac screening for athletes around the world. In some countries such as Italy, this is compulsory.7,10,14 Many other European countries have followed suit in accordance with the ESC recommendations, and several professional sporting bodies, such as the International Olympic Committee (IOC)38 and Fédération Internationale de Football Association (FIFA),39 recommend cardiac screening.
The incidence of sports related sudden cardiac arrest is low, 6–7/million inhabitants per year in one recent study.40 Interestingly, it showed only 5.3% occurred in young competitive athletes, with the remaining occurring in middle-aged recreational sports participants. Only 12% had a history of heart disease.
The causes in the young, below 35 years, include cardiomyopathy, coronary artery anomalies, ion channelopathies, and acquired cardiac conditions with geographical variation in incidences.41 In individuals who are older, CAD accounts for >80% cases with untrained individuals appearing to be at the highest risk.42 Screening of recreational middle-aged sports participants for underlying coronary artery disease may be more beneficial in changing behaviours and reducing the burden of sudden cardiac arrest.42
Risk profiling
Current NICE recommendations suggest a systematic strategy within primary care to identify individuals at the highest risk of CVD.43 This should be reviewed regularly in those over the age of 40 years. Well-validated risk scores such as QRisk344 in the UK and SCORE in Europe aid this and help determine the need for primary preventative interventions. Patients with chronic kidney disease, albuminuria, Type 1 diabetes, or familial history of hypercholesterolaemia should be assumed to be high-risk and treated accordingly.42
The U.S. Preventive Services Task Force (USPSTF)45 has similar recommendations. They advise against screening asymptomatic individuals with a low-risk of CVD using ECG or exercise ECG and find insufficient evidence for a recommendation in intermediate- or high-risk. Their recent review on screening for AF in asymptomatic adults aged 50 years or older found insufficient evidence to assess the benefits versus harms.46 This is consistent with the recent LOOP study,47 where, despite a nearly three-fold rise in AF detection and subsequent anticoagulation, there was only a non-significant trend to benefit. This was coupled with a non-significant trend to harm such as major bleeding. This suggests that the correct demographic to screen has yet to be found.
Screening of sudden cardiac arrest survivors and victims’ families
A standardised approach would help phenotype and genotype individuals and has the potential to do more for our understanding of rare cardiac conditions capable of causing SCD than any whole population screening programme could ever detect. The ESC’s 2015 guidelines suggest that a diagnosis could be made in 50% of families of sudden arrhythmic death victims.48 There are guidelines from the European Society of Pathology (ESP) for autopsy investigations of victims of SCD.49
For survivors of sudden cardiac arrest who come under the care of cardiology, a thorough evaluation of the cause of arrest should be made before discharge to determine the need for implantation of a cardio-defibrillator and other therapies. However, some survivors never come under the care of a specialist cardiology team and the opportunity to screen family members is lost.49
Serendipitous Screening
With the huge rise in the use of cross-sectional radiological imaging over the last 10 years, there has been increasing recognition by radiologists that as they can see the heart, they should analyse it too. Calcification of the coronary arteries is easily visible on both unenhanced and enhanced studies and there have been several papers in recent years guiding how to best interpret and deal with these findings.50
The degree of coronary calcification increases with age, as does the likelihood of having a CT with at least some of the heart visible. Other signs of coronary heart disease can also be visible, such as left ventricular wall scarring, late enhancement of the myocardium, mural, and intracardiac thrombus. CT scanners are now so fast that these features are often visible on non-gated studies.
Now that Schrödinger’s ‘coronary cat’ has been irreversibly observed, the radiologist must decide how to report it without creating unnecessary demand on cardiology services. Estimating the calcium score is feasible but should be put in the clinical context of the patient. For example, a male in their 50s with three-vessel calcification has a clear risk that might benefit from investigation and treatment. A 95-year-old male with metastatic malignancy may not.
The British Society of Cardiovascular Imaging (BSCI) published a consensus statement on this in 2020, which detailed how best to approach incidental cardiac findings.50 They suggest interpretation should be influenced by additional available clinical information. A similar approach is made to aortic valve calcification and other incidental cardiac findings. Reports will alert the clinician to the presence of disease and having a strategy to deal with this is important. Most of the follow up should be suitable for the family physician, with symptomatic disease most likely to require onward referral. A useful pathway exists in their document referring to NICE guidelines.50
Digital Screening
With the worldwide increase in usage of mobile devices, there exists a basis for a digital health approach in the context of arrhythmia, be it as a diagnostic tool or for surveillance. This can be particularly beneficial in the context of AF, where the incremental costs for its use are relatively low.51
Several digital devices are available to diagnose and record heart rhythm changes. Among these is the MyDiagnostick (MyDiagnostick Medical, Maastricht, the Netherlands), a device equipped to record a single-lead ECG that displays a red or green light if AF or sinus rhythm is detected. AF analysis has shown 80–100% sensitivity and 93–99% specificity and a screening study during flu vaccinations found 1.1% of participants had AF.52 The Zenicor-ECG (Zenicor Medical Systems, Stockholm, Sweden) is another hand-held device with no additional hardware. Two electrodes at each end are held and the central display shows a Lead I ECG. AF was identified in 0.9% of participants in one screening study and 3% in another study, both adopting similar protocols. Validity was high when used twice daily along with recordings during symptoms and adjudication of ECGs by a health professional.53,54
The RhythmPad (Cardiocity, Colchester, UK) is a mousepad-style ECG screening device55 that offers an advantage over the single-lead view, as a third electrode can be added for enhanced image clarity. The titanium-based sensors can be placed around both arms and the right leg then attached via leads to a tablet computer that displays a six-lead ECG. Among the advantages over single-lead systems, is the existence of algorithms for the detection of arrhythmias beyond AF. A study utilising it revealed sensitivities of 97.5% for normal sinus rhythm and 95.4% for AF.55
The Zio Patch (iRhythm Technologies, San Francisco, California, USA) provides continuous ECG recording for 14 days with a high diagnostic yield for total arrhythmia detection when compared with Holter monitoring.56 When 24 hours of monitoring was compared between the two methods, the Holter detected more arrhythmias; however, the time to first recorded arrhythmia often occurred after 48 hours, demonstrating the importance of longer duration monitoring. Comfort is an important consideration and impacts compliance. Both the Zio Patch and the similar S-PATCH (Wellysis, Seoul, Republic of Korea) were found to be superior in this regard when compared with traditional Holter ECG monitors.56-58
Commercial wearable devices measure heart rate and rhythm through ECG or PPG systems. ECG monitors can be built into belts, wristbands, adhesive patches, and mobile smartphones. PPG measures changes in microvascular blood volume that translates into pulse waves and a tachogram recording. This technology is advancing, and more arrhythmias are becoming identifiable.59 Diagnostic clarity can be enhanced by over-reading from a competent practitioner in ECG analysis. As well as issues with sensor contact, challenges include signal correlation and patient comfort. Future developments should focus on overcoming such design barriers.
One wearable device, the Apple Watch (Apple, Cupertino, California, USA), has gone some way towards achieving both comfort and accuracy and was evaluated in the Apple Heart Study.20 This showed that 2,161 people had an irregular rhythm, with 34% confirmed with AF on subsequent patch monitoring. Where AF was not the cause of rhythm irregularity, 40% showed other arrhythmias, mostly ectopic beats.20,60 Adverse events were collated and anxiety was recorded most commonly, supporting findings that such devices can cause health anxiety through overuse and is worthy of consideration when considering such devices with patients.20,61
Perhaps the most widely adopted tool in AF screening research is the Kardia device (AliveCor, Mountain View, California, USA), a device that transmits a single-lead ECG wirelessly to a smart mobile device. The NICE recently published their guidance on the Kardia, outlining the cost-effectiveness and ability to identify significantly more AF than Holter monitoring.62 A systematic review explored the feasibility and validity of the device.63 Feasibility metrics, including process, resource, and management, revealed this as an effective tool. Sensitivity and specificity both reached 98% across included studies, with AF detection ranging from 0.8% to 36%, with correlation to the study demographics and screening approach.63 Kardia has also demonstrated utility across a variety of settings, making it versatile and easy to use. Their recent six-lead version offers advantages over the single-lead view, with the addition of more sophisticated algorithms including corrected QT interval ECG analysis.
Remote monitoring of cardiac implantable electronic devices is now recommended by major cardiology societies.64,65 There has been an increase in use over the last few years and advantages include earlier detection of events and identification of device malfunction, permitting earlier intervention. Enhanced patient safety, reducing hospital admissions, and improving quality of care whilst proactively identifying problems contributes to the cost savings. The increasing need for monitoring patients has come at a time when there is clear evolution and improvement in the accuracy and efficacy of digital health devices.
DISCUSSION
Overall, there are multiple ways of screening for cardiac disease. In most diseases, the exact population that may benefit from screening has yet to be identified. The rapidly expanding field of smart devices and the use of AI may help identify these groups further and provide information about disease trends. Digital devices have the potential to screen large sections of the population for multiple types of pathology. It opens the possibility of examining previously impossible data, such as a patient’s heart rhythm prior to their first collapse. It has the potential to reduce the cost of screening, especially in more remote areas. Various devices may also help monitor the middle-aged starting to exercise, with their potential increased risk of cardiac disease.
Personal devices such as the Apple Watch empower patients to look after their own health and to control their own data. This can occasionally be associated with increased health anxiety but, conversely, can also enable reduction of a patient’s unease. For example, patients can use devices such as the Kardia whenever they get palpitations. There is also the expanding field of AI, especially in combination with imaging. This shows great promise at increasing diagnostic accuracy and assisting the risk stratification of patients.
There are of course negatives aspects to health screening, which will need to be weighed against the benefits. The wide-reaching screening that some of these digital devices might provide could be used very broadly, perhaps to identify a suitable screening target population. Digital health devices are still in adolescence with multiple unknowns; however, the future looks promising.
CONCLUSION
In conclusion, the expanding field of digital health devices has the potential to offer multiple new methods for screening for heart disease. There needs to be some caution to ensure that these technologies are properly evaluated to comply with the principles of screening, especially when comparing the potential harms and benefits of their use.








