Abstract
Background: Cardiac valve calcifications (CVC) are common among patients on haemodialysis (HD). The valves most commonly involved are mitral and secondarily aortic valves. In Libya, there is a lack of research in this field. This is the author’s motivation to conduct this study, which has a significant impact on the health status of patients on HD.
Objective: To identify aortic valve calcifications (AVC), prevalence of aortic stenosis (AS) in patients on HD, and to determine clinical aspects and risk factors that may lead to the development of AS.
Patients and Methods: A cross-sectional study was conducted between May–November 2023 of adult patients on chronic HD who had received haemodialysis for more than 1 year. Patients with previous cardiac surgery, a history of endocarditis, or severe anaemia (haemoglobin <7.5 mg/dL) were excluded. A sample of 48 patients were enrolled in the study, with all patients undergoing clinical, biochemical, and a trans-thoracic echocardiographic evaluation.
Results: Patients were aged 31–60 years ±13.2 standard deviation. AS had a prevalence of 6.3%, mitral regurgitation had a prevalence of 33.3%, and CVC was detected in 60.4% of patients on chronic HD, with AVC seen in 52.1% and mitral valve calcification seen in 25%. Patients with AVC were more often females (58.6%). Diabetes was seen in 64.6% of cases, and autosomal polycystic kidney disease was seen in 25%. The authors found that dyspnoea was the most common symptom (66.7%), followed by palpitations (35.4%) and asymptomatic patients (27.1%). Clinically detected AS was observed in three individuals (6.3%). Surprisingly, patients did not exhibit significant differences in age, duration of dialysis, or comorbidities. However, hyperphosphatemia was detected in 56.25% of patients, and hyperparathyroidism was recorded in 64.50%.
Conclusion: The study has shown that aortic stenosis is the second most common valvular lesion in patients on chronic HD, preceded by mitral valve regurgitation. However, asymptomatic AVC has the highest prevalence among patients on chronic HD. Hyperphosphatemia and hyperparathyroidism are major risk factors that enhance the calcification of cardiac valves.
Value of the Research: Though it is a snapshot study, it addresses an important comorbidity in patients on chronic HD. Namely, prevalence of aortic stenosis, CVCs, and associated risk factors, which had been reported in many regional and global nephrology literature, but the national Libyan literature still lacks such chronic HD patients’ data.
Key Points
1. Cardiac valve calcifications are common among patients on haemodialysis. The most commonly involved are mitral and secondarily aortic valves.
2. This cross-sectional study addresses an important comorbidity in patients on chronic haemodialysis, exploring important risk factors contributing to the increased prevalence of these cardiac lesions in this sector of patients.
3. This study is a forward step in Libyan nephrology literature, encouraging more active and long-term research from other institutes in Libya.
INTRODUCTION
Cardiovascular disease is still the leading cause of death in patients on haemodialysis (HD).1 Cardiac valve calcification (CVC) is expected to occur 10–20 years earlier in patients with chronic kidney disease (CKD) than in the general population, and its progression is estimated to be 10 times faster in patients on HD.2,3 Aortic valve calcification (AVC) varies between 44–55% in patients on chronic HD.4 Calcified aortic valve disease results from the deposition of calcium nodules on the aortic valve, lipid/inflammatory mediators, as well as anatomic and genetic mechanisms unique to aortic valves causing endothelial injury, valve thickening, and functional stenosis.5,6,7 This degenerative process is attributed to additional factors in uremic patients, such as uremic milieu with retained toxins, loss of calcification inhibitors that promote intimal and medial vascular calcification, metabolic bone disease, chronic hypertension, and volume overload, which are also pathogenic factors in such population.8 Aortic stenosis (AS) in patients on HD has a progressive calcific degeneration of valve leaflets, when compared with patients without CKD.9,10 Symptomatic aortic stenosis depends on the severity of valve lesion, and the typical symptoms include dyspnoea and presyncope. However, patients on HD may present with dialysis-related hypotension, dialysis intolerance, peridialysis atrial arrhythmias, and post-dialysis fatigue.11,12 The authors’ research covers the prevalence of aortic stenosis and any associated CVCs in patients on HD attending Benghazi Nephrology Center, linked with clinical aspects of AS, and explores the risk factors that may lead to the development of aortic stenosis in patients on HD.
The main objectives of this study were to determine the prevalence of AS and CVCs in patients on chronic HD, determine the clinical aspect of AS, and identify risk factors that may lead to the development of AS and CVCs in patients on HD.
PATIENTS AND METHODS
Study Design
This is a cross-sectional (case-series) echocardiography-based prospective study conducted with patients on chronic HD; utilising echocardiography performed by four independent cardiologists.
Sampling
A sample of 48 patients on chronic HD, selected by convenience sampling, was completed between May 2023–November 2023. Patients were subjected to a clinical questionnaire and transthoracic echocardiography on a non-haemodialysis day to determine the presence of CVCsor lesions.
Inclusion and Exclusion Criteria
The inclusion criteria was patients on haemodialysis for >1 year and aged >18 years. Exclusion criteria included previous cardiac valve surgery, history of endocarditis, or patients who were severely anaemic (haemoglobin <7.5 gm/dL) to avoid overlap with ostensible cardiac valve lesion.
Clinical Questioner and Interview
Using a self-structured questionnaire, reported information was collected on name, age, and duration of dialysis. Medical chart reviews were conducted to determine comorbidities such as a history of diabetes, dyslipidaemia, atherosclerotic cardiovascular disease, or any other chronic medical condition. Current symptoms like dyspnoea, palpitation, chest pain, or intermittent claudication were recorded. In addition, general and cardiac examinations were done to evaluate patients at the beginning and end of the study period, patients were then referred to the Centre’s cardiologists on a non-dialysis day to perform transthoracic echocardiography. Relevant laboratory data were recorded, including patients’ haemoglobin, parathyroid hormone (PTH), calcium, and phosphorus levels over the study period.
Statistical Analysis
Data were recorded, stored, and prepared for analysis. All statistical analyses and data processing were performed using the Statistical Package for Social Sciences (SPSS) version 28 software (IBM, New York, USA). Data presented as mean ± standard deviation (SD) for quantitative variables and summarised as frequencies and percentages for categorical variables. For inferential statistics, variables were analysed by chi-squared test, and a p-value of <0.05 is considered a statistically significant finding.
Ethical Statement
This study has been approved by the Libyan International Medical University ethics research committee (ERC) and was issued an ethical approval code: MDC-2023-00129. All questionnaires were anonymous and unidentified to ensure the confidentiality of the collected information. Respondents have provided written consent to both answer the questions and to participate in the study.
RESULTS
The study recruited 48 patients (20 [41.7%] male and 28 [58.3%] female) on chronic HD. Patients’ age ranged from 31–60 years ±13.2 SD. The majority of patients (n=29, 60.4%) were on chronic HD for 1–5 years, while 10 (20.9%) patients were on chronic HD for more than 10 years patients; the mean duration of HD was 6.48 years ±5.16 SD. The primary causes of end-stage renal disease were diabetes (n=31, 64.6%), autosomal dominant polycystic kidney disease (ADPKD; n=12, 25.0%), and unknown CKD (probably chronic glomerulonephritis; n=5, 10.4%). Diabetes is more common in females (n=18, 58.1%) than in males (n=13, 41.9%). While ADPKD is more common in male patients (n=8, 66.7%) than female patients (n=4, 33.3%). This study has shown that hypertension has a high prevalence, being present in 44 patients (91.6%), due to poor compliance with medications. Exploring dialysis sessions from a clinical point of view, the authors found that dyspnoea is the most common complaint among the cohort of patients, being recorded in 32 patients (66.7%). Palpitation was recorded in 17 patients (35.4%) and chest pain in four patients (8.3%). It is worth mentioning that dyspnoea is more common in males than females (p=0.023), while asymptomatic dialysis sessions are more common in females than males (p=0.024; Table 1). Forty-eight patients were subjected to transthoracic echocardiographic study by independent cardiologists. Aortic valve calcification was detected in 25 patients (52.1%), while AS was detected in three patients (6.3%). Mitral valve calcification was detected in 12 patients (25%), while mitral regurgitation (MR) was detected in 16 patients (33.3%). The details of the echocardiographic findings are depicted in Table 2. When reviewing patients’ available laboratory data profiles during the study period, the authors found that the patient cohort had mean values of serum calcium 9.98±3.31 mg/dL, serum phosphorus 5.18±1.66, intact PTH 863.43±138.20 pg/mL, and haemoglobin 10.49±2.35 g/dL, indicating disturbed mineral bone homeostasis, with no statistically significant gender differences. Meanwhile, vitamin D2 levels were found in a few patient records (n=8, 16.6%), with a marginal mean value of 28.6±5.68 ng/mL (Table 3).
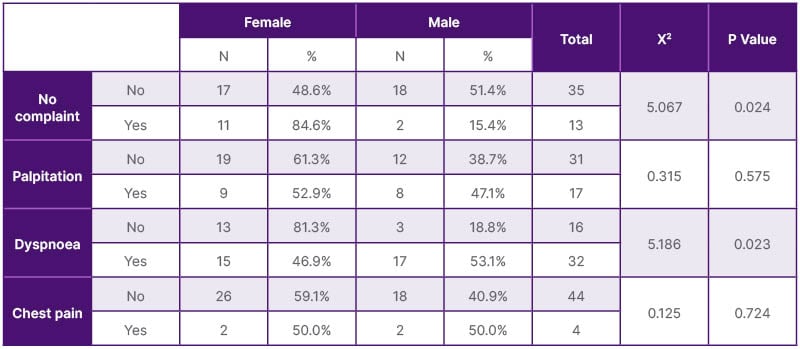
Table 1: Clinical aspects of dialysis sessions.
X2: Chi-square test.
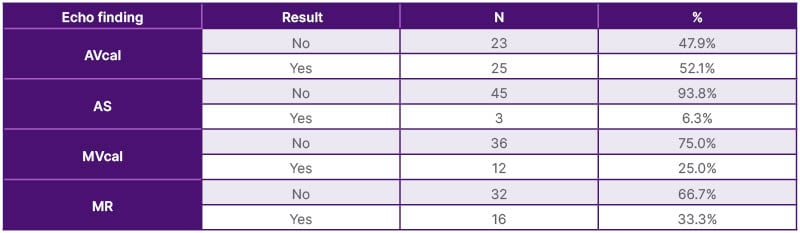
Table 2: Echocardiograph findings in patients on chronic haemodialysis and their impact on cardiac valves.
AVcal: aortic valve calcification; AS: aortic stenosis; MR: mitral regurgitation; MVca;: mitral valve calcification; N: number.
DISCUSSION
Despite advances in HD technology, AS and CVC are considered a high prevalence comorbidity in patients on chronic HD, occurring 4–5 times more commonly and in a shorter time frame than in the general population. They are anticipated to be surrogate markers of future coronary artery disease.13,14 In the UK, it is estimated that 1.5% of the over-55-year-old population has AS.15 Vascular calcification is defined as a form of calcium-phosphate complexes deposition in the vasculature, mainly including intimal calcification, Mönckeberg medial arterial calcification, and valvular calcification.16 In a recent Kidney Disease: Improving Global Outcomes (KDIGO) 2019 report, the prevalence of aortic valve calcific abnormalities ranged from 28–85%; however, AS (as opposed to aortic calcification) was present in 9.5% of patients with CKD, compared with 3.5% of the general population, with similar patterns for mitral regurgitation (43% versus 24%, respectively).17-19 It is prudent to identify these cardiac lesions in patients on chronic HD.
In this cross-sectional echocardiography-based study, patients’ age ranged from 31–60 years ±13.2 SD. Of the 48 patients, 20 (41.7%) were male and 28 (58.3%) were female. This matter differs from region to region, with males starting chronic HD earlier than females in some countries. In a multicentre study conducted in Serbia from 2014–2019, there had been a relative propensity of males on HD, with male patients making up about twice that of female patients throughout the 5 years investigated.20 Another larger multicentre study, conducted in 12 countries (across Europe, Asia, and the USA), has shown that, in all age groups, more males than females were on haemodialysis (59% versus 41% overall, respectively), with large differences observed between countries. The male-to-female mortality rate ratio in the general population varied from 1.5–2.6 for age groups <75 years, but in patients on chronic HD the ratio was close to one.21
The majority of patients (n=29, 60.4%) in the authors’ study had a duration of HD between 1–5 years (mean 6.48 years ±5.16 SD), but it was found to not be statistically significant. In a study from Morrocco, a single-centre cross-sectional descriptive and analytical study of 111 adult patients who were on chronic HD for more than 6 months in 2013, the average age of patients was 44 years ±14 SD.22 The average duration of haemodialysis was 146 months ±80 SD, and a univariate analysis showed that haemodialysis duration was associated with the occurrence of CVCs, approaching a marginal level of significance (p=0.09).22 A recent metanalysis in 2023 has shown that older age (odds ratio: 1.09; 95% CI: 1.06–1.12) and longer duration of dialysis (odds ratio: 1.08; 95% CI: 1.01–1.16) were significantly associated with a higher prevalence of cardiovascular calcifications.23
In the authors’ study, diabetes was the most prevalent comorbidity (64.6%), followed by ADPKD (25%). A Korean epidemiological study covering 2002–2017 showed that diabetes is still the most common aetiology of an incident in patients on HD, steadily increasing from 23.84–47.84% over a 15-year study period.24 In a Chinese study published in 2016, patients with diabetes had a higher CVC and coronary artery calcification score, and higher incidence rate of major adverse cardiovascular events.25 It has been shown in the current study that dyspnoea is the most common symptom (66.6%) in patients with moderate-to-severe AS or MR, followed by palpitations (35.4%). However, patients with a milder degree of cardiac valve lesions exhibited tolerance during dialysis sessions, were more commonly asymptomatic (27.0%), and had no record of chest pain manifestation during this study (Table 1).
In a 2021 cohort study conducted in Japan on 35 patients on chronic HD with a median follow-up of 4 years, exercise intolerance in the form of shortness of breath was found in 34%, syncope in 14%, and chest pain in 20% of patients with AS.26 Echocardiographic assessment of the current authors’ cohort of patients showed that AS was observed in 6.25% patients; aortic valve calcification was detected in 52.1% of patients, with modest female predominance (58.6%); MR was detected in 33.3%; and mitral valve calcification occurred at a rate of 25% (Table 2). In a retrospective echocardiography-based study from Singapore including 4,119 patients with severe AS (aortic valve area <1 cm2) from 2000–2018, researchers detected severe CKD in 230 patients (5.5%) and found that females constituted 58.3% of patients (versus 58.3% in this study).27 It also found that severe AS was present is in 34±4% of patients with severe CKD; however, the Singaporean patients were relatively older (80 years ±8 SD), compared to this study cohort of patients (31–60 years ±13.2 SD). Diabetes was recorded in 41.7%, compared to 64.6% in the current study.27,28
The degree of aortic valve leaflet calcification is a strong predictor of AS development. In a retrospective multicentre study involving 1,507 patients on chronic HD, the median time for progression from one to two or three leaflets calcification was 3–4 years.29 The adjusted hazard ratios (95% CI) of two- and three-leaflet calcified were 2.12 (1.49–3.00) and 4.43 (3.01–6.52), respectively, compared to one-leaflet calcification.29 When exploring potential risk factors for CVCs, the authors found that most of their patients (n=37, 77.1%) were normo-calcemic (mean 9.98±3.3 SD; P=0.092), and 27 patients (56%) had serum phosphorus levels of >4.5 mg/dL, being hyperphosphatemia (mean 5.18 ±1.66 SD; p=0.558); a result that has been confirmed in several studies. In Libyan literature, the prevalence of hyperphosphatemia in patients on chronic HD was found to be 68%.30 A retrospective study in Japan, conducted over 10 years and including 441 patients on chronic HD, found a significant correlation between high phosphate levels and the occurrence of AS, and mandates annual echocardiographic screening for these patients.31,32 However, the prevalence of secondary hyperparathyroidism has been studied in the Libyan dialysis population, with prevalence ranging from 17.0–27.1%.30,33 In this study, the authors found that serum intact PTH was high (>200 pg/mL [mean 863.43 ±138.20 SD; p=0.29]) in 31 patients (64.5%), which defines a high prevalence of a state of secondary hyperparathyroidism in the patient cohort (Table 3).34 The lack of statistical significance of these biochemical findings is attributed to a smaller sample size.
Gender differences contribute as risk factors for AS in patients on chronic HD, with male patients being more prone to accelerated cardiac valves and coronary calcifications, and it is predicted that testosterone probably plays a role in accelerated calcifications.35 In this study; the female-to-male ratio was 1.3:1, which is a marginal finding. Furthermore, no racial differences exist in the study, since all patients are native Libyans. However, several reports show racial differences do exist. In comparison to Black patients, White patients with advanced CKD may have a more rapid progression of AS.36,37 Though it is beyond the authors’ current research, it is worth mentioning that CVCs were more frequently found in haemodialysis compared to peritoneal dialysis patients.38 The preservation of residual renal function, and less shear stress on valve leaflets in peritoneal dialysis patients, might be making the difference compared to patients on haemodialysis. Furthermore, peritoneal dialysis patients may have a greater time-averaged exposure to phosphate clearance than haemodialysis.39
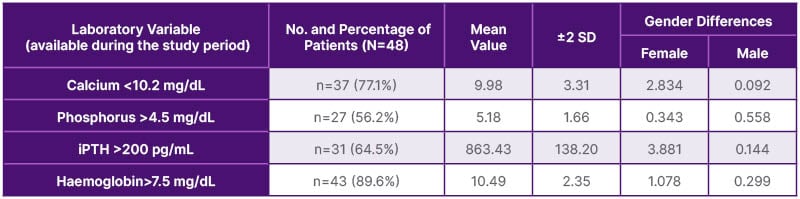
Table 3: Laboratory data available during the study period.
iPTH: intact parathyroid hormone; No: number; SD: standard deviation.
CONCLUSION
The prevalence of AS is high (6.3%) in this cohort of Libyan patients on HD. The prevalence of CVC among patients on HD was observed in 60.4%, aortic valve calcification in 52.1%, mitral valve calcification in 25%, and mitral regurgitation was recorded in 33.3% of patients. The authors observed that the majority of patients had a duration of 1–5 years on chronic HD therapy, stating that the cardiac calcification process had already started during their CKD stages. Dyspnoea was the most common symptom in patients with AS. On the other hand, it was found that gender difference is marginal. A majority of patients in the study had high phosphorus and intact parathyroid levels, though this was not statistically significant, probably due smaller sample size. Age and duration of dialysis were not found to be significant risk factors.
RECOMMENDATIONS
The authors suggest an echocardiography screening programme for AS in high-risk individuals, such as middle and old-age patients on chronic HD.40 They also recommend having strict control of hyperphosphatemia and secondary hyperparathyroidism in patients onchronic HD.







