Abstract
Since the first balloon angioplasty in 1977, remarkable advances in catheter-based technology have been achieved with the use of stents and intracoronary devices. However, despite these developments, visual assessment of a 2-dimensional lumenogram of the coronary vessels remains the predominant method of assessing coronary disease and guiding angioplasty worldwide. It is an enigma that there is still such a low uptake in the use of intravascular imaging, whether in the form of intravascular ultrasound (IVUS) or optical coherence tomography (OCT), with both techniques providing cross-sectional imaging of the coronary vessels with a resolution at the microscopic scale. The intracoronary imaging community tends to focus on the academic aspects of IVUS and OCT, often highlighting the evidence-based benefits in lesion subsets, such as left main stem or bifurcation percutaneous coronary intervention. However, this does not impart crucial practical-related aspects pertaining to IVUS or OCT, which can improve outcomes and achieve optimal results for operators and patients. Here, the authors present a case-based approach to IVUS and OCT use in contemporary clinical practice, with the hope of providing useful, practical insights for the busy interventionalist and prompting the consideration of intracoronary imaging catheters as an essential part of their percutaneous coronary intervention toolbox.
INTRODUCTION
For >50 years, coronary angiography has been the gold standard for the assessment of coronary anatomy; however, as an isolated technique to guide the management of ischaemic heart disease, its limitations are well known. Cardiologists are now able to move beyond a simple 2-dimensional visual assessment of the vessel lumen, using complementary techniques that deliver cross-sectional imaging of the entire coronary vessel and provide essential information to guide coronary intervention.
Intracoronary imaging is not a recent innovation; it first led to a paradigm shift in stent implantation and optimisation in 1995 when the switch from an anticoagulation to an antiplatelet strategy in percutaneous coronary intervention (PCI) with the aid of intravascular ultrasound (IVUS) was first described. This switch led to reduced bleeding rates that are still seen during antiplatelet therapy today.1
Of the imaging technologies that are widely available, IVUS is the most well established; however, more recently, optical coherence tomography (OCT) has also progressed into day-to-day clinical practice. Both techniques deliver additional information that a lumenogram of coronary angiography alone cannot provide, although the use of these techniques varies widely across the world.2 In the UK, IVUS is used in 7% of PCI cases and OCT in 2%,3 compared to IVUS use in >80% of cases in Japan.4
Traditionally, reviews of intracoronary imaging tend to focus on the academic and technical aspects related to IVUS or OCT use. Here, a case-based approach to understanding intracoronary imaging use is presented from the practical perspective, using clinical situations that will be familiar to any interventional cardiologist in 2018.
THE TECHNOLOGY
Both IVUS and OCT rely on a probe mounted on a catheter, which is advanced over a standard 0.014-inch angioplasty wire and then withdrawn mechanically or manually by operator pullback across the area of interest. The probe emits ultrasound waves during IVUS or near-infrared light during OCT, and the reflected waves are used to construct a cross-sectional image of the vessel. Ultrasound, with a frequency of 20–60 Hz, depending on the commercial device, allows an axial resolution of 40–150 µm (Figure 1D) with a penetration of 4–8 mm.5 In contrast, OCT provides a higher axial resolution of 10–20 µm, at the expense of a reduced penetration of 1.0–2.5 mm.6 Advancements in technology now allow co-registration of images (IVUS-angiography or OCT-angiography), removing any operator error in translating the cross-sectional imaging to a location on angiography. Tri-registration with instantaneous wave-free ratio, IVUS, and angiography is also available, allowing true multimodality anatomical and physiological assessment of coronary lesions.
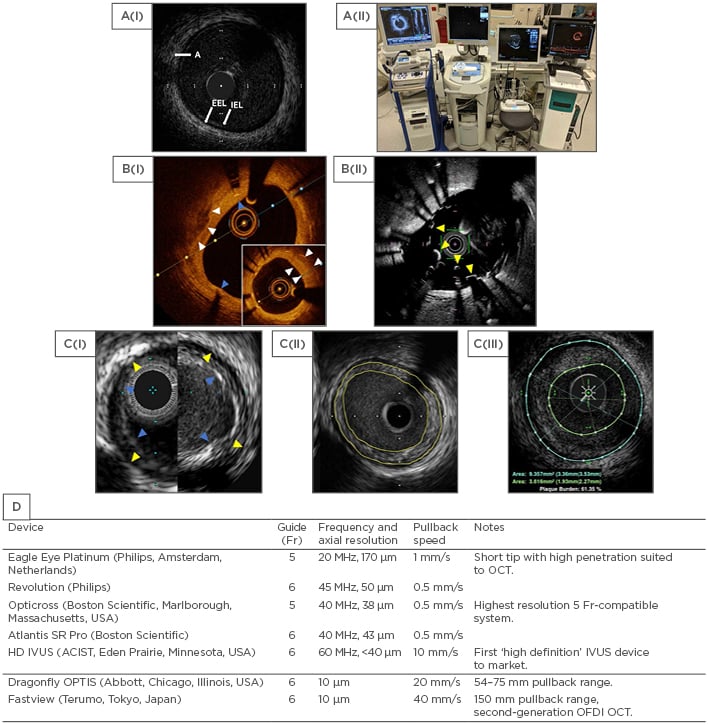
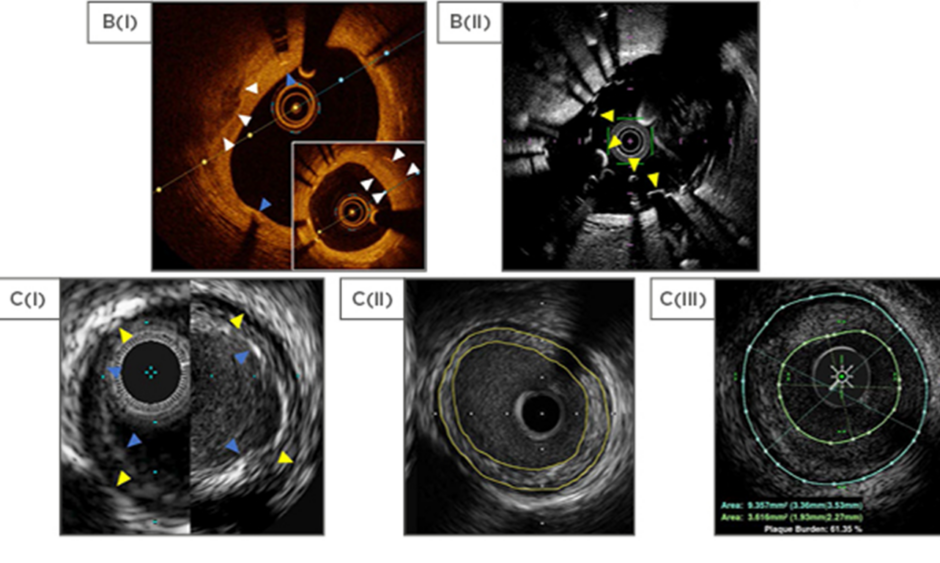
Figure 1: Comparison of intravascular ultrasound and optical coherence tomography technologies.
A(i): Normal coronary vessel by HD IVUS (ASCIST). A(ii): Stand-alone intracoronary imaging consoles, left to right: Philips (formerly Volcano), Boston Scientific, ACIST HDI, Abbot (formerly St Jude Medical). B(i): OCT (OPTIS) view of calcified plaque (white arrows) with overlying stent struts and stent shadows (blue arrows). The distal vessel shows significant neointimal formation (insert, white arrows). B(ii): OCT (Fastview) view of an underexpanded stent (yellow arrows) that was not apparent at angiography. C(i): Comparison between Philips Eagle Eye Platinum (20 MHz, left) and Revolution (45 MHz, right). EEL as yellow arrows and IEL as blue arrows. C(ii): Concentric plaque outlined in yellow (Boston, Atlantis SR Pro). C(iii): Concentric plaque by IVUS outlined in green and blue (ACIST, HD IVUS). D: Currently available IVUS/OCT catheters in Europe.
A: adventitia; CTO: chronic total occlusion; EEL: external elastic lamina; HD: high definition; IEL: internal elastic lamina; IVUS: intravascular ultrasound; OCT: optical coherence tomography; OFDI: optical frequency domain imaging.
Intravascular Ultrasound
IVUS catheters are compatible with ≥5 Fr guide catheters and are either mechanical rotating or phased array in design. In a rotating design, the transducer rapidly rotates to produce higher resolution images, whereas for a phased array device the transducer is fixed. Automated pullback is available for most devices at a speed of up to 10 mm/s. The generated greyscale image allows assessment of the entire vessel wall and identification of the internal elastic lamina, external elastic lamina, and adventia (Figure 1A).
To overcome the limitations of greyscale IVUS, complementary technologies have been developed to further analyse atherosclerotic plaques. Near-infrared spectroscopy in combination with IVUS is available as a single catheter (Infraredx, Inc., Burlington, Massachusetts, USA) and uses near-infrared light to determine plaque composition. The chemogram generated can be displayed as a colour-coded ring around the standard IVUS image to indicate areas with a high concentration of lipids. Dedicated device software automatically calculates a lipid core burden index, with higher values reflecting lipid-rich plaques.
Radiofrequency IVUS also combines greyscale IVUS with further analysis to provide information about coronary plaque composition. Proprietary software is available from Volcano, San Diego, California, USA (virtual histology, VH-IVUS); Terumo Medical Corporation, Tokyo, Japan (integrated backscatter-IVUS); and Boston Scientific, Marlborough, Massachusetts, USA (iMAP™).7
Optical Coherence Tomography
An OCT catheter contains a single fibre optic core rotating within a transparent sheath that emits light across a range of wavelengths. OCT offers high spatial resolution and clear images, offset by reduced tissue penetration compared to IVUS. In addition, there are technical differences that must be taken into consideration with the use of OCT; for example, red blood cells scatter the light emitted by the probe and therefore OCT requires the artery to be clear of blood. Typically, this is achieved by flushing the vessel with contrast, which can be of concern in patients with renal impairment. Rapid automated pullback is available, allowing imaging of the vessel up to a speed of 40 mm/s, which is faster than IVUS catheters (10 mm/s). In addition, the high-contrast images of OCT (Figure 1B) show reduced interobserver and intraobserver variability compared to greyscale IVUS.8
CLINICAL CASES
For any patient who comes to the catheter laboratory, the operator must ask themselves: ‘Does this patient have a mandate for intervention?’. Once this decision has been made, they must consider a wealth of information before deciding on the revascularisation strategy. Identifying the lesions that require treatment; whether the lesion requires preparation; the type, length, and number of balloons and/or stents; and how to optimise stent deployment can be made possible with the aid of intracoronary imaging. The following four cases illustrate instances when the use of intracoronary imaging has proved key in diagnosis and management.
Case 1
A 35-year-old male smoker who works as a local chef presented with a 2-hour history of central chest pain. Prehospital 12-lead ECG showed lateral ST-elevation and the patient was brought directly to the cathlab. The right coronary artery was unobstructed; however, angiography of the left system revealed a hazy lesion within the mid left anterior descending artery (LAD) (Figure 2A(i)).
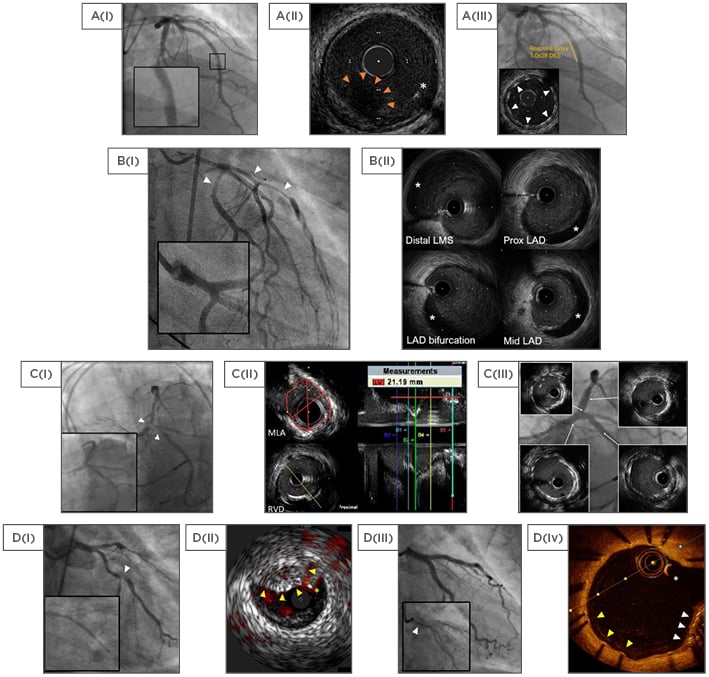
Figure 2: Selected cases using intravascular ultrasound and optical coherence tomography.
A: Plaque rupture; A(i): Hazy mid LAD magnified; A(ii): 60 MHz HD IVUS showing plaque rupture with thrombus (hypoechoic with red arrows) and guidewire (asterisk); A(iii): Final result with well-expanded struts (arrows). B: Spontaneous coronary artery dissection; B(i): Impaired flow angiographically (arrows), with the distal left main stem suggestive of dissection (magnified); B(ii): 40 MHz IVUS (Atlantis SR Pro) images with the false lumen (hypoechoic, asterisks). C: LMS intervention; C(i): Severe LMS disease (arrows) compared to previous angiography (insert); C(ii): MLA and RVD measurements with automated pullback; C(iii): Final angiographic result with IVUS (inserts). D: Stent failure; D(i): LCx bifurcation disease (arrow) with failure to advance the stent (magnified); D(ii): IVUS with coregistered ChromaFlo highlighting blood red (Eagle Eye, Philips), used to guide wire placement. Unexpanded stent (yellow arrows) and guidewire (asterisk); D(iii): Final result with the crushed stent (magnified, arrow); D(iv): Surveillance OCT showing the guidewire (asterisk), crushed stent (white arrows), and adequate stent coverage (yellow arrows).
HD: high definition; IVUS: intravascular ultrasound; LMS: left main-stem; MLA: minimum lumen area; OCT: optical coherence tomography; Prox: proximal; RVD: reference vessel diameter.
At this stage, the diagnosis by angiography alone was uncertain. The differential diagnosis included coronary embolisation, spontaneous coronary artery dissection (SCAD), and plaque disease, though one would suspect atherosclerotic disease to be less likely given the patient’s age and absence of ischaemic risk factors. An IVUS catheter was advanced, followed by manual pullback across the lesion; this showed normal coronary arteries distally and proximally, with plaque rupture and adherent fresh coronary thrombus at the site of angiographic abnormality (Figure 2A(ii)) in the mid vessel. An Export aspiration catheter (Medtronic, Minneapolis, Minnesota, USA) was passed, followed by stenting with a RESOLUTE ONYX™ (Medtronic) 3.5×22.0 mm stent and post dilation (the final result of which can be seen in Figure 2A(iii)). The patient was treated with dual antiplatelet and statin therapy, and was discharged 4 days later.
Case 2
A 32-year-old female with no past medical history presented with 1 hour of central chest pain. Prehospital 12-lead ECG showed anterolateral ST-elevation with reciprocal ST-depression and she was brought directly to the cathlab for angiography. The right coronary system was free of any disease. The left coronary system showed an abnormal proximal circumflex (LCx) and abnormal proximal LAD involving the first diagonal (Figure 2B(i)). The patient’s pain and ECG changes resolved with the administration of intracoronary nitrates.
Possible diagnoses at this stage included coronary spasm, given the response to nitrates, or a recanalised infarct. Transthoracic echocardiography showed anterolateral hypokinesia with preserved left ventricular systolic function, and her initial troponin T was 1,100 ng/L (reference: <14 ng/L). The patient was treated with dual antiplatelet therapy and abciximab. Further review of the images suggested distal left main-stem (LMS) dissection flap (Figure 2B(i), magnified).
IVUS re-study confirmed SCAD involving the distal LMS, proximal LAD, and first diagonal (Figure 2B(ii)). The patient was managed medically with dual antiplatelet therapy and discharged 5 days later. Surveillance angiography at 10 weeks confirmed complete resolution of the dissection.
Culprit Lesion Identification and Characterisation
The majority of myocardial infarction cases are due to atherosclerotic plaque rupture or plaque erosion, the site of which may not be apparent following coronary angiography. In those with unremarkable coronary arteries, occult plaque rupture was identified as the cause of myocardial infarction in 38% of patients, but only after the use of IVUS.9 These are patients who may otherwise have been managed for myocarditis or thrombophilia, treated for coronary spasm, and/or denied the benefits of dual antiplatelet and statin therapy. In Cases 1 and 2, intracoronary imaging clearly identified the aetiology as atherosclerotic plaque rupture and SCAD, respectively.
OCT has been shown to be superior to both IVUS and coronary angioscopy in identifying plaque rupture, erosion, and coronary thrombus.10 This ability to differentiate between plaque erosion and plaque rupture may provide future opportunities for risk stratification and targeted therapy in acute coronary syndrome (ACS).11,12 Indeed, it has been suggested that in ACS patients with plaque erosion identified by OCT, antithrombotic therapy alone may be a viable alternative to stenting.13 Finally, OCT also provides a good alternative to IVUS for the detection of dissection. OCT was not used in these cases due to concerns regarding propagating a SCAD false lumen. However, it should be noted that guidelines suggest that the use of OCT is safe in this situation.14
Case 3
An 80-year-old female with a background of transcatheter aortic valve replacement and permanent pacemaker implantation 2 years prior, PCI to LAD 7 years prior, chronic kidney disease stage 4, and peripheral vascular disease presented with exertional chest pain. Coronary angiography revealed severe distal LMS, severe ostial LAD disease, and severe ostial LCx disease; this was a significant progression from the coronary angiography conducted 3 years prior (Figure 2C(i)). The initial revascularisation strategy pursued was PCI of the LMS into the LAD with the T and protrusion technique, and a provisional T stent for the obtuse marginal branch.
IVUS revealed a ring of fibrocalcific disease not evident on angiography. Measurements showed a reference vessel diameter of 5.5 mm, minimum lumen area (MLA) of 3.08 mm2, and lesion length of 21 mm. After preparation with compliant and cutting balloons, a Xience Prime 3.5×23.0 mm stent (Abbott, Lake Bluff, Illinois, USA) was inserted. The LCx was stented without complication followed by usual kissing balloon inflation. The result is shown in Figure 2C(iii), with IVUS demonstrating well-expanded stents throughout.
Case 4
A 49-year-old transgender female with a history of hypertension and smoking presented with several hours of intermittent chest pain. The initial 12-lead ECG showed anterolateral ST-elevation and the patient was brought directly to the cathlab; on arrival, she was in cardiogenic shock.
The LAD was occluded at the level of the first diagonal with Thrombolysis in Myocardial Infarction (TIMI) 0 grade flow (Figure 2D(i)). The LCx had severe ostial disease, as well as severe disease at the bifurcation with a large marginal branch. Following thrombus aspiration, the LAD was predilated and stented from the mid vessel to ostium without difficulty. The patient’s anterior ST-elevation resolved; however, she still had persistent lateral ST segment elevation at this stage.
An attempt was made to direct stent the LCx and marginal branch with a 3.5×38.0 mm Ultimaster® stent (Terumo Medical Corporation). However, the stent could not be advanced into the LCx and on withdrawal of the device, the stent came free from the balloon; an attempt to readvance the balloon and inflate the stent in a suboptimal position was unsuccessful. Rewiring the stent with a Fielder XT (Asahi Intecc, Nagoya, Japan) wire under IVUS guidance was also unsuccessful (Figure 2D(ii)). Therefore, the trapped stent was crushed into the adventitia using sequential balloon inflation, followed by stenting from the marginal back into the LMS. IVUS showed underexpansion in the LMS and proximal LCx; therefore, proximal optimisation was carried out with a 4.0×12.0 mm noncompliant balloon at 25 atm. The patient was discharged from hospital after 3 days and surveillance OCT at 3 months showed good stent coverage throughout (Figure 2D(iv)).
Stent Optimisation
The most common use of intracoronary imaging is for stent optimisation. Cases 3 and 4 illustrate the benefit of intracoronary imaging in lesion preparation and ensuring adequate stent expansion. Although a somewhat unusual situation, Case 4 also highlights the application of IVUS in directing guidewire passage, though this is more often seen in the context of chronic total occlusion recannalisation.
While several criteria to define optimal stent deployment by intracoronary imaging have been published, including by the authors of this review,15 no consensus currently exists. All criteria aim to reduce the known predictors of adverse events: stent underexpansion, incomplete stent apposition, and geographical miss (failing to completely cover the lesion or edge dissection). When used for this indication, intracoronary imaging modifies the revascularisation strategy in over one-third of patients versus angiographic guidance alone.16 In addition, this use of intracoronary imaging is also associated with reduced hard clinical endpoints of stent thrombosis, target lesion revascularisation, myocardial infarction, and cardiac death versus an angiography-only strategy (Table 1).17-20 This has been demonstrated best in patients with left main disease, long lesions, or chronic total occlusion, rather than by routine use in all cases.18,21
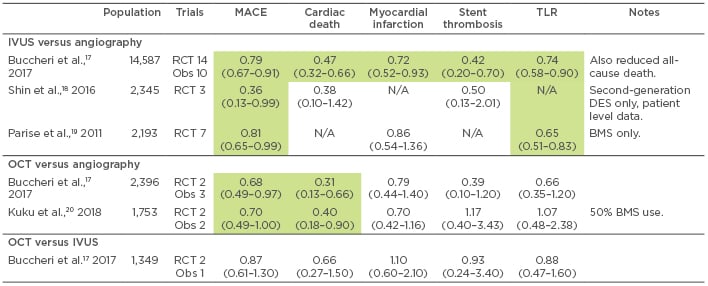
Table 1: Selected meta-analyses of intravascular ultrasound and optical coherence tomography-guided angioplasty.
Green indicates significantly reduced risk versus comparator. All results as odds ratio (95% confidence interval), unless specified, for IVUS or OCT versus comparator.
BMS: bare metal stent; DES: drug-eluting stent; IVUS: intravascular ultrasound; MACE: major adverse cardiovascular events; N/A: not available; OCT: optical coherence tomography; Obs: observational; RCT: randomised controlled trial; TLR: target lesion revascularisation.
Though the use of IVUS is well established for stent optimisation, OCT provides an attractive alternative given its enhanced resolution and that its main limitation (reduced penetration) is of less concern. Results from increasing numbers of trials involving OCT are becoming available, with ILUMIEN III22 and OPINION16 published in the last 2 years. Both studies showed the non-inferiority of OCT versus IVUS-guided PCI with regard to minimal lumen area and clinical target vessel failure, respectively. However, OPINION lacked an angiography-only arm and large, long-term studies powered to demonstrate a difference in clinical outcomes are lacking. This may change with ILUMIEN IV,23 which is currently recruiting to compare OCT versus angiography in up to 3,650 patients with coronary artery disease and high-risk or complex lesions, who possibly stand to benefit the most from the high-resolution imaging that OCT offers.
Stent Surveillance
The higher resolution of OCT is particularly suited to evaluation of stents after implantation, as in Case 4 (Figure 2D(iv)), with the assumption that stent coverage represents neoendothelialisation. In addition, OCT is appropriate for evaluating the mechanisms behind stent failure. It has been suggested that OCT-defined features, such as tissue heterogeneity, tissue backscatter, and visibility of microvessels, may provide a histological correlation of the vascular response to stenting,24 though this may not be true outside the setting of stent restenosis.25
OTHER APPLICATIONS
Left Main Stem Assessment
Much effort has been made to assess lesion haemodynamic severity by intracoronary imaging. However, the potential disparity between lesion anatomy and ischaemia means assessment of haemodynamic significance remains firmly in the domain of physiological indices, such as fractional flow reserve (FFR) and the instantaneous wave-free ratio.26-30
Neither IVUS nor OCT have shown any binary cut-off measurements suitable for justifying coronary intervention. The exception to this is LMS disease, for which there is a reasonable correlation between MLA by IVUS and FFR.31 The proposed MLA threshold for intervention has decreased in recent years, with the suggestion that a MLA as low as 4.5 mm2 in the right population may correlate well with a FFR ≤0.8.32 Nonetheless, current guidelines recommend a cut-off of 6 mm2 for intervention, which has shown acceptable sensitivity for significant LMS disease in outcome-driven studies.33,34
IVUS may be used in LMS and bifurcation stenting to ensure adequate stent expansion. In Case 4, underexpansion was only apparent with the use of IVUS. Ensuring a MLA >5 mm2 at the ostium of the LCx, >6.3 mm2 at the ostium of the LAD, and >8.2 mm2 in the main body of the LMS has been associated with reduced rates of in-stent restenosis and major adverse events.35 Similar cut-off values in determining LMS significance and stent optimisation were also recently used in a large trial of contemporary PCI versus coronary artery bypass surgery.36
By comparison, while assessment of the LMS by OCT is technically feasible,37 there are some specific challenges. The low penetration of OCT limits assessment of plaque burden, and, in ostial LMS lesions, precise positioning of the guide catheter to achieve an adequate blood-free field can be difficult.38 No data currently support the use of OCT in determining LMS (or any other epicardial vessel) lesion significance, and IVUS is likely to remain the modality of choice for this indication for the foreseeable future.
High-Risk Plaque
Intracoronary imaging allows analysis of atherosclerotic plaques on a lesion-by-lesion basis, and the arterial remodelling process in response to the stresses of atherosclerosis may provide an indicator of high-risk lesions. Outward growth of the external elastic membrane with preservation of the luminal area (positive remodelling) in the early stages of atherosclerosis leads to increased plaque vulnerability and may explain why positive remodelling and only modest stenoses are often evident in ACS. In comparison, vessel shrinkage and loss of luminal area (negative remodelling) are more common in more stenotic yet stable lesions.39
Although intracoronary imaging has been extensively studied with regard to identifying a ‘vulnerable plaque’ (particularly VH-IVUS) and supported by data from the PROSPECT trial,40 it has not progressed to day-to-day clinical use. The main reason for this is that sufficient information about plaque characteristics can often be obtained from correct interpretation of the greyscale IVUS images; therefore, the addition of VH-IVUS may not alter the treatment strategy. In the PROSPECT trial,40 which investigated lesion-related risk factors, the strongest predictor of future lesion events was a plaque burden of ≥70% (odds ratio: 4.99; 95% confidence interval: 2.54–9.79). VH-IVUS-identified thin-capped fibroatheroma and a MLA ≤4.0 mm2 were also reasonable predictors (odds ratio: 3.00 and 2.77, respectively). The follow-up event rate at a median of 3.4 years was 10% when there was a plaque burden ≥70% and MLA ≤4.0 mm2. The addition of VH-IVUS-identified thin-capped fibroatheroma increased this rate to 17%.40
Despite this, it is suspected that most interventionalists are not confident enough to correctly identify thin-capped fibroatheroma on VH-IVUS given the infrequent use of this technique. As a result, from a practical standpoint, measurement of the greyscale plaque burden, symmetry index, and MLA at various points along the vessel lumen may suffice. Indeed, no lesion events occurred in PROSPECT trial patients with <40% plaque area involvement.41
Zero-Contrast Angiography
Given the limitations of angiography, with a maximum of two orthogonal views using biplane imaging per contrast injection, intracoronary imaging offers the potential to reduce or eliminate the use of radiocontrast. This is an attractive proposal for those with renal insufficiency or severe contrast allergy and has been performed successfully with IVUS.42,43 Case reports show that this may also be possible with OCT, though problems with colloid nephrotoxicity remain.44,45
GUIDELINES
Guidelines and consensus statements have been published by the major European and American societies for the use of IVUS and OCT during revascularisation.34,46-48 The use of IVUS to optimise stent implantation in selected patients, as well as in the assessment and optimisation of LMS treatment, both carry IIa recommendations from the European Society of Cardiology (ESC). American and European guidelines advise that IVUS and OCT should be considered to detect the mechanisms behind stent failure; however, these guidelines are now several years old and updated ESC guidelines are due for publication in 2018.
CONCLUSION
Intracoronary imaging is a widely available and essential adjunct to angiography, allowing cardiologists to deliver the best possible outcomes for their patients. While there are monetary and healthcare system barriers to the widespread adoption of intracoronary imaging worldwide, the authors hope that this review will help interventional cardiologists recognise the proven benefits of intracoronary imaging and encourage them to reach for the IVUS or OCT catheter more often in day-to-day clinical practice.