Abstract
Coronary artery spasm is an abnormality of coronary vascular smooth muscle contraction that is associated with significant morbidity and mortality. The underlying pathophysiological process has remained unclear since Myron Prinzmetal described it in 1959. This article reviews current literature of the pathogenesis and outlines clinical features, diagnosis, and treatment options.
INTRODUCTION
Coronary artery spasm (CAS) is caused by abnormal coronary artery vascular smooth muscle (VSM) contraction.1 The pathophysiological process underlying CAS remains uncertain, leading to numerous incongruous descriptions of this disease. In 1959, Prinzmetal et al.2 first realised variant angina as being a separate entity from classical angina pectoris, described by Heberden.3 Prinzmetal et al. described a syndrome independent of physical exertion, during which ST-segments are transiently and often remarkably elevated but almost always terminate spontaneously. It was thought that this transient myocardial ischaemia was brought on by temporary occlusion of a large diseased artery due to an increase in vascular wall tone.3
The advent of coronary angiography has allowed for direct visualisation of the coronary arteries during episodes of CAS. This demonstrated patients with angiographically unobstructed coronary arteries,4,5 leading to the idea that variant angina represented focal spasm of coronary arteries in otherwise disease-free vessels.6 More recently there has been discussion that CAS occurs at the site of atherosclerotic plaque lesions.7
EPIDEMIOLOGY
The prevalence of CAS is difficult to characterise due to variation within different populations, variable use of provocative testing, and differing diagnostic criteria. CAS is more prevalent in males, with most patients aged between 40–70 years, but this prevalence tends to decrease after the age of 70 years.8,9 Japanese populations have a greater frequency of CAS10 (thought to be secondary to hyperreactive coronary arteries) when compared with their Caucasian counterparts.11 Western studies have demonstrated, in selected population groups, the prevalence of CAS amongst patients with angiographically unobstructed coronary arteries (no focal obstruction >50%) undergoing provocative testing at 49% and 33.4%, respectively.12 In addition, the frequencies of multiple regions of spasm during provocation testing in Japanese (24.3%) and Taiwanese (19.3%) populations are higher than those of Caucasian populations (7.5%).13
Although not supported by robust epidemiological data, the incidence of CAS is thought to be declining.14 The decreased incidence of smoking is thought to be a major contributing factor, along with the increased use of calcium channel blockers (CCBs)15 for hypertension, and statins for primary and secondary prevention of ischaemic heart disease. There is also thought to be a reduced interest in performing time-consuming provocative testing in the cardiac catheterisation laboratory14 and a presumed diagnosis of CAS is normally managed with a trial of CCBs.
CLINICAL FEATURES
CAS can manifest clinically as a wide spectrum of coronary syndromes, which can make diagnosis challenging. It is suspected in the presence of severe chest pain at rest, with concurrent electrocardiogram (ECG) changes showing transient ST-segment elevation. Although chest pain remains the most common symptom of CAS, it is worth noting that many attacks of CAS can be asymptomatic or silent.16 Syncope may occur during these silent ischaemic episodes.17 A distinguishing feature of CAS is that angina is thought to occur predominantly at rest and not during exercise; this has preponderance for occurring late at night or early in the morning, with a peak frequency reported as 5 am.18,19 Interestingly, light exercise, when performed early in the morning, has been shown to induce episodes of spasm, whereas strenuous exercise in the afternoon did not provoke the same episodes.20 Therefore, CAS is thought to follow diurnal circadian variations possibly related to the contributions of the autonomic nervous system (ANS).
DIAGNOSIS
Electrocardiogram
Although Prinzmetal’s angina is most commonly associated with ST-elevation, the ECG findings of CAS are extremely varied and may in fact be absent. Traditionally, a total or subtotal spasm of a major coronary artery will lead to ST-elevation in the corresponding distribution of that artery. CAS is also frequently associated with ST-segment depression rather than ST-elevation.21 There are a number of associated arrhythmias including sinus bradycardia, sinus arrest, atrioventricular block, paroxysmal atrial fibrillation, ventricular tachycardia, ventricular fibrillation, and asystole, lending further credence to the argument that CAS encompasses a spectrum of diseases and can present in a wide variety of ways. Sudden death may also result from CAS, with bradyarrhythmia rather than tachyarrhythmia being the most common terminal event.22,23
CORONARY ANGIOGRAPHY
Yasue et al.16 suggested that CAS can be diagnosed clinically if the anginal attacks disappear quickly upon administration of nitroglycerin and any one of five specific criteria are met: attacks appearing at rest in the morning, diurnal variation in exercise tolerance, presence of transient ST-elevation, attacks induced by hyperventilation, or ability of CCBs to suppress the attacks.
Angiography may reveal normal, or near normal, coronary arteries.24,25 The presence of angiographically normal coronary arteries in CAS has been widely documented in studies from Japan.16 This may be due to environmental and genetic factors that have been suggested to give a higher prevalence of CAS in the Japanese population.1,10 More recently it has been suggested that the presence of normal coronary arteries is actually overstated and that atherosclerotic disease, in the form of focal irregularities along the coronary vasculature, contributes to almost all focal CAS.14 This is supported by evidence of focal irregularities detected by intravascular ultrasound in angiographically ‘normal’ coronary arteries.26,27 Therefore, the higher proportion of normal coronary arteries seen in the Japanese population may be a result of a lower threshold for describing a coronary artery as ‘normal’ on angiography.
PROVOCATIVE TESTING
The diagnosis of CAS should ideally be made by witnessing a CAS angiographically during an attack, however this is often not possible. Provocative testing allows for the induction of CAS by certain physiological processes or pharmacological agents in order to demonstrate the vasculature’s abnormal predisposition to contraction. Hyperventilation,28 exercise testing,29 and cold pressor tests have all been used to physiologically induce CAS, however these are limited by modest sensitivity. Ergonovine and acetylcholine are the most commonly used agents for this purpose and cause contraction of smooth muscle cells that are more sensitive to these agents due to the presence of endothelial dysfunction.30-33 Although provocative testing with ergonovine or acetylcholine has good sensitivity for the detection of CAS,34 associated complications have resulted in a decline in utilisation, especially in Europe and North America.35 However, a recent study demonstrated that when performed in controlled environments, the use of acetylcholine provocation testing can be safe and effective at demonstrating diffuse CAS in patients with unobstructed coronary arteries (<50% obstruction).35,36
PATHOPHYSIOLOGY
The causes of CAS are multifactorial and remain poorly defined. Much has been made of the potential role of the ANS in the development of spasm, yet the relationship remains complex and not fully explained. The parasympathetic system was initially thought to be central to CAS as it has preponderance for occurring at night when vagal tone is highest,37,38 and acetylcholine is known to induce CAS.16 However, alpha adrenergic vasoconstriction by the sympathetic nervous system has also been suggested as a possible trigger for CAS.39,40 Propranolol has been shown to exacerbate spasm, presumably because of the resultant unopposed alpha adrenergic vasoconstriction.41 Despite the above evidence, therapies aimed at manipulating the effects of the ANS have failed to translate into effective treatments.42,43
Endothelial dysfunction, although not always present in patients with CAS,44 is thought to be central to the hyperreactive vasomotor response of coronary arteries. In ‘normal’, healthy individuals, acetylcholine causes arterial vasodilatation,45 however in vessels prone to spasm it causes abnormal constriction and hence is used in provocation testing.35 Ergonovine and acetylcholine both cause vasodilatation in normal vessels via the release of nitric oxide, therefore dysfunctional endothelial nitric oxide synthase has been suggested as a possible contributor to CAS.46 Underlying atherosclerotic lesions are often the substrate on which these changes in the endothelium take place. Statins and vitamin E have both been shown to improve endothelial function and reduce symptoms of CAS.47,48 Smoking is a known risk factor for CAS and may contribute to the development of endothelial dysfunction through chronic low-grade inflammation.49 High levels of circulating inflammatory cytokines have been shown to be independently associated with a diagnosis of CAS,50 and chronic inflammation of the vascular endothelium is thought to predispose arteries to abnormal spasm.51
The effectiveness of CCBs at reducing the frequency of symptoms of CAS may be directly linked to the hypercontractility of VSM in affected arteries. Animal models have suggested a role for increased Rho kinase activity which can directly increase the sensitivity of myosin light-chain to calcium, augmenting its phosphorylation and thus favouring contraction of the smooth muscle cell.52 Other animal models have suggested a number of pathways through which hypercontractility in smooth muscle cells may be induced, however their relevance remains to be clarified.53,54
TREATMENT
In the acute setting, early treatment of CAS is important to the prevention of significant complications such as myocardial infarction, arrhythmias, and sudden cardiac death. Sublingual, intravenous, or intracoronary nitroglycerin in the acute phase has been shown to be effective for relieving attacks (Figure 1).55
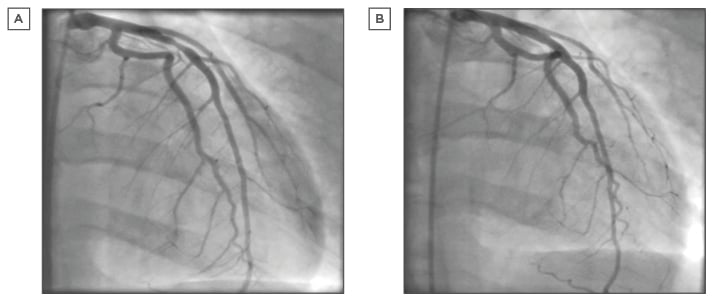
Figure 1: Coronary angiogram.
A) Left coronary system demonstrating significant obstructive proximal LAD artery stenosis secondary to coronary spasm; B) Left coronary system after intracoronary nitrate demonstrating resolution of proximal LAD artery stenosis.
LAD: left anterior descending.
Cessation of smoking is the most effective non-medical intervention for the prevention of further attacks of CAS. As CAS is likely to occur in the context of existing atherosclerotic disease, controlling risk factors for ischaemic heart disease must also be undertaken to try and control recurrent episodes of spasm.49 Other non-medical treatments include avoiding drugs which induce spasm, reducing alcohol intake,56 avoiding emotional stress, and replacing any magnesium deficiency.1,57,58
CCBs have demonstrated efficacy at reducing the frequency of attacks of CAS by inhibiting the contraction of VSM.59 The use of CCBs has been shown to be an independent predictor of survival and can successfully reverse provocative testing, allowing for the safe discontinuation of medical treatment if necessary.60 Interestingly, complications of CAS such as atrioventricular block have been terminated with CCB therapy.61 High-dose long-acting CCBs are used as the initial treatment and are uptitrated according to symptomatic response, with the occasional requirement of using two agents to control symptoms. Given the preponderance for attacks of CAS to take place at night and in the early hours of the morning, CCBs should be taken at night before bed to be most effective.1
Long-acting nitrates, despite not having any prognostic benefit, also act at the level of the VSM to promote relaxation and prevent episodes of spasm. They have not been shown to be as effective as CCBs and their use is limited by their side effect profile62 and the development of tolerance.63 Despite this, they can be used safely in combination with CCBs to try and suppress recurrent episodes of spasm. Nicorandil, a potassium channel activator, may also be used in the management of recurrent episodes of spasm refractory to CCBs or nitrates.64 Other agents, including magnesium, antioxidants, Rho kinase inhibitors, statins, and pioglitazone65 have all been suggested as possible treatments for CAS due to their effects on vascular endothelial function, however their integration into clinical practice remains to be seen.
Non-medical treatment of CAS has included attempts at using angioplasty and coronary artery bypass grafting to try and prevent further episodes of spasm. The reports of cases treated with coronary artery bypass grafts (CABG) have given variable results.66 The success of this operation in the treatment of CAS is most likely dependent on the degree of atherosclerosis present and to what extent that disease is contributing to the spasm. If there is a focal site of spasm caused by atherosclerotic disease, then it could be effectively bypassed. However, if the spasm is multivessel and diffuse, CABG would be less efficacious. In order to address this problem, one approach considered was to perform a sympathectomy to prevent diffuse spasm. This seems to improve the outcome. Sympathectomy has recently been used in the treatment setting of acute CAS causing life-threatening complications.67
Primary percutaneous coronary intervention (PCI) has been presented as another possible treatment for refractory CAS.68 PCI may provide a rapid and effective treatment for focal spasm that presents as a life-threatening complication. Unfortunately, the long-term outcomes following PCI remain variable.69 Once again this most likely represents the wide spectrum of underlying pathology in CAS. Some cases demonstrate the diffuse spasm of multiple arteries, which are clearly not suitable for a targeted intervention such as PCI. Each case must be considered individually and the appropriate non-medical treatment applied depending on the nature of the spasm (diffuse or fixed), the possible underlying atherosclerotic disease, and the acuteness of the presentation. More research is required and a randomised controlled trial should be performed, investigating the possibility of PCI as a treatment for CAS. (Table 1)59,60,69-76
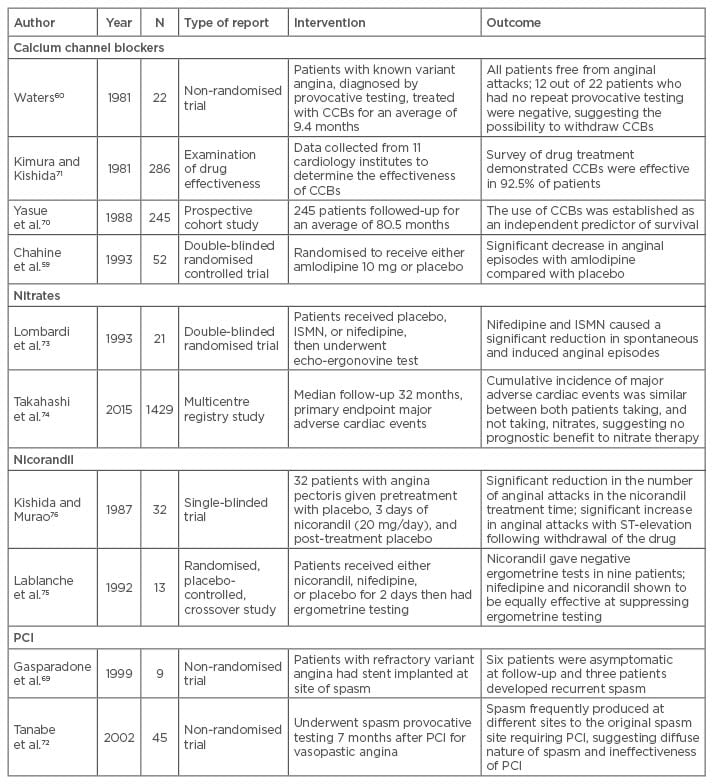
Table 1: Treatment for coronary spasm.
CCBs: calcium channel blockers; ISMN: isosorbide-5 mononitrate; PCI: percutaneous coronary intervention.
PROGNOSIS
Major adverse cardiovascular events (including death and myocardial infarction) for CAS are difficult to quantify due to the lack of consensus in diagnostic criteria. Initial studies reported 3-year major adverse cardiovascular events rates between 5% and 37%. However, more recent studies have described rates of ≤1%. Of note, the period of highest major adverse cardiovascular event rates is within 3 months from onset of symptoms. This highlights the importance of early recognition and diagnosis, especially in patients presenting with acute coronary syndromes without culprit lesion, unexplained syncope, or aborted sudden cardiac death.7 The Japanese Coronary Spasm Association (JCSA) risk score can be utilised for risk assessment and prognostic stratification of CAS patients.77
CONCLUSION
CAS is caused by abnormal coronary artery VSM contraction and can manifest clinically as a wide spectrum of coronary syndromes, which can make diagnosis challenging. The prevalence of CAS is difficult to characterise due to variations within different populations. CAS is more prevalent in males, with most patients aged 40–70 years. The Japanese Coronary Spasm Association risk score can be utilised for risk assessment and prognostic stratification of CAS patients. CAS is suspected in the presence of severe chest pain at rest, with concurrent ECG changes showing transient ST-segment elevation. Chest pain remains the most common symptom of CAS, although silent attacks can occur, for example with syncope.
The causes of CAS are multifactorial and remain poorly defined. The ANS and endothelial dysfunction are thought to play an integral role in the pathophysiology of CAS. Treatment of CAS includes sublingual or intravenous nitroglycerin in the acute phase. In addition, long-acting nitrates, CCBs, and nicorandil all have a role in the treatment of CAS. There is limited evidence of invasive therapy with PCI or CABG, which should be considered in refractory cases.








