Chairpeople: Yvan Vandenplas1,2, Hania Szajewska3
Speakers: Rosan Meyer,4,5,6 Katerina Bajerova,7,8 Yvan Vandenplas1
1. KidZ Health Castle, University Hospital Brussels, (UZ Brussel), Belgium
2. Free University of Brussels (VUB), Brussels, Belgium
3. Department of Pediatrics, Medical University Warsaw, Poland
4. Imperial College London, UK
5. Winchester University, Hampshire, UK
6. Katholieke Universiteit (KU) Leuven, Belgium
7. Department of Pediatrics, University Hospital, Brno, Czechia
8. Masaryk University, Brno, Czechia
Disclosure: Vandenplas has participated as a clinical investigator, advisory board member, consultant, and/or speaker for Abbott Nutrition, Biogaia, By Heart, CHR Hansen, Danone, ELSE Nutrition, Friesland Campina, Nestlé Health Science, Nestlé Nutrition, Nestlé Nutrition Institute, Nutricia, Mead Johnson Nutrition, Phathom Pharmaceuticals, United Pharmaceuticals (Novalac), and Wyeth. Szajewska has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for: Arla, BioGaia, Biocodex, Danone, Dicofarm, Nestlé Nutrition, Nestlé Health Science, Nestlé Nutrition Institute, Nutricia, and Mead Johnson. Meyer has received honoraria for academic lectures, served on advisory boards, and/or received research grants from Abbott, Danone/Nutricia, Mead Johnson, Else, Nestlé Health Science, and Nestlé Nutrition Institute. Bajerova has received honoraria from Nutricia/Danone and Nestlé Health Science.
Acknowledgements: Writing assistance was provided by Helen Boreham, Yorkshire, UK.
Support: The symposium and publication of this article were supported by the Nestlé Nutrition Institute and Nestlé Health Sciences.
Citation: EMJ Allergy Immunol. 2022;7[Suppl 1]:2-10. DOI/10.33590/emjallergyimmunol/10179590. https://doi.org/10.33590/emjallergyimmunol/10179590.
Meeting Summary:
CoMiSSTM is a clinical tool developed to increase awareness among healthcare professionals (HCP) of possible symptoms of cow’s milk allergy (CMA) in infants. During this symposium, leading experts in the field of paediatric gastroenterology, allergy, and nutrition highlighted how CoMiSS can facilitate awareness of CMA and support HCPs in improving the patient journey from symptom presentation to diagnosis. Rosan Meyer, Imperial College London, UK; Winchester University, Hampshire, UK; and Katholieke Universiteit (KU) Leuven, Belgium, summarised the major challenges of CMA diagnosis, which underscore the need for improved clinical tools to increase HCP awareness of hallmark symptoms. Katerina Bajerova, Katholieke Universiteit (KU) Leuven, Belgium, and Department of Pediatrics, University Hospital, Brno, Czechia, reviewed the current evidence base for CoMiSS and presented key learnings from recent clinical experience using this tool. Yvan Vandenplas, KidZ Health Castle, University Hospital Brussels (UZ Brussel), Belgium, showcased the latest updates to CoMiSS for 2022 proposed by the expert consensus panel and explained how these improvements would help increase the application of CoMiSS in raising CMA awareness.
Why is the Diagnosis of Cow’s Milk Allergy So Challenging?
Rosan Meyer
CMA is one of the most common food allergies of early childhood, and evidence suggests that an estimated 1.8–7.5% of infants are affected.1 In 2015, the EuroPrevall birth cohort study of 12,000 infants found an overall prevalence of CMA in Europe of 0.54%, ranging from <0.3 – 1.3% across different countries.2 The inter-country disparities and low rates of non- IgE-mediated allergy identified in this birth cohort have raised some concerns about the clinical recognition of this delayed-type CMA, commented Meyer.3
The well-known Diagnosis and Rationale for Action Against Cow’s Milk Allergy (DRACMA) guidelines divide CMA into two main groups of conditions associated with IgE and non-IgE-mediated reactions to cow’s milk.4 To facilitate their recognition by clinicians, these conditions have been ‘translated’ into symptoms. Symptoms broadly impact the gastrointestinal (GI) tract, respiratory tract, and skin, in addition to the more severe systemic symptoms.5-8 The challenge is that these commonly seen symptoms in CMA can also be found in myriad other conditions, noted Meyer.
An excellent example is an overlap between functional paediatric GI disorders, eczema, and CMA. A recent paper evaluating a cohort of 650 breastfed infants from the EAT study documented the incidence of vomiting and colicky symptoms at 22% each and eczema at 43% (Figure 1). Overall, 13% of this cohort showed other symptoms related to CMA, but only 0.7% had a challenge-proven allergy.9 These results align with global figures on the prevalence of functional GI disorders in infants, with regurgitation reported in 30% and colic in 20%, irrespective of food allergy status.8 The concern around using symptoms alone as a diagnostic tool was further highlighted in the EAT study’s secondary analysis of 1,303 infants. In this analysis, 1 in 11 infants displayed two or more severe International Milk Allergy in Primary Care (iMAP)-defined symptoms of CMA, while three-quarters had two or more ‘mild/moderate’ symptoms.10
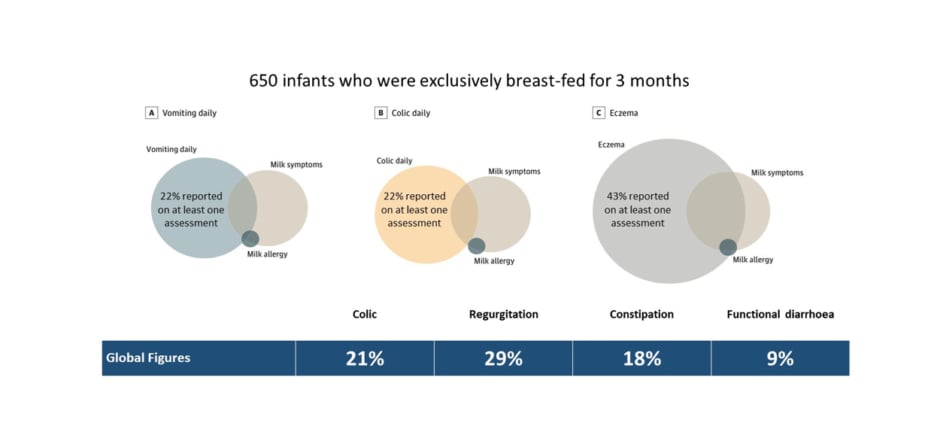
Figure 1: Overlap between common infant symptoms and cow’s milk allergy in the EAT study.8,9
Particular challenges lie in the diagnosis of non-IgE-mediated allergy, emphasised Meyer. In addition to overlapping symptoms, symptom onset may be delayed and the association between food and symptoms is often ambiguous, particularly in infants during complementary feeding who are consuming multiple allergens. No reliable tools are currently available for diagnosing non-IgE-mediated allergies outside of endoscopy, so clinicians must rely on the fallible process of food elimination and reintroduction.
As well as potential symptom-based overdiagnosis of CMA, there is equal concern surrounding the poor recognition of CMA and delays in diagnosis in many cases. Health economic data from the UK have revealed a gap of 2.2 months from symptom presentation to the initiation of treatment, with an average of 2‒6 general practitioner (GP) visits required before arriving at a final CMA diagnosis.11 This delay in diagnosis does not go without consequences, Meyer stressed. Underdiagnosis can have a detrimental impact on the child’s own health, leading to faltering growth and an increased risk of micronutrient deficiencies.12 It also exerts a negative toll on the family and broader healthcare systems.11,13 As Meyer elaborated, the health economic burden imposed by recurrent physician visits, inappropriate prescribing of hypoallergenic formula, and multiple feed changes on the pathway to correct diagnosis and treatment of CMA is often forgotten.
It is, therefore, critically important for clinicians to strike the correct balance between over- and underdiagnosis of CMA. GPs are typically “the first port of call” for parents of children with CMA symptoms, explained Meyer; however, a 2015 survey of over 400 GPs revealed that the majority are unfamiliar with leading guidelines in the field.14 Overall, 18%, 55%, and 81% of surveyed GPs admitted they were ‘not aware’ of guidelines on CMA from the National Institute for Health and Care Excellence (NICE), iMAP, or European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), respectively (Table 1). These findings highlight the need to increase awareness and provide tools to aid front-line HCPs, including physicians, dieticians, and nurses, in diagnosing CMA, concluded Meyer.
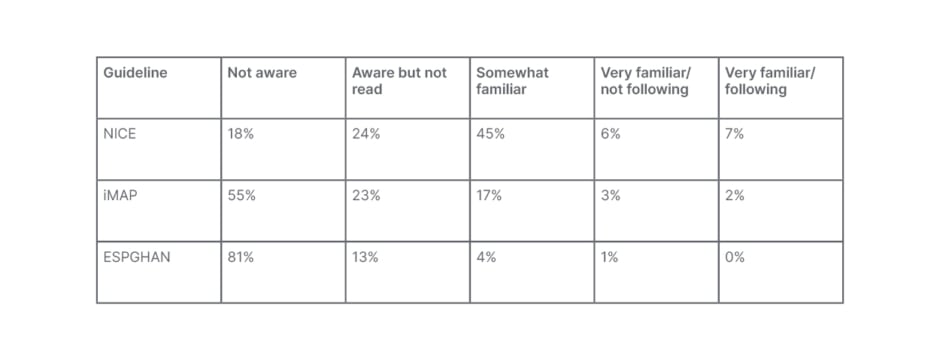
Table 1: Shortfalls in general practitioner knowledge of current cow’s milk protein guidelines.14
ESPGHAN: European Society for Paediatric Gastroenterology, Hepatology and Nutrition; iMAP: International Milk Allergy in Primary Care; NICE: National Institute for Health and Care Excellence.
A systematic review of symptom scores carried out in 2020 to inform the triage for CMA pinpointed two potential diagnostic tools: the CoMiSS awareness tool and the Gibbons et al. 2012 symptom score.15-18 CoMiSS was initially developed to assess symptoms for a multicentre trial on two different hypoallergenic formulas, after which (with input from a group of international experts) it evolved into the original version of CoMiSS. Meyer described CoMiSS as a simple symptom awareness tool aimed at increasing HCP recognition of CMA and noted that, since its original identification, it has undergone extensive study and validation. In total, since 2015, 25 original research studies using CoMiSS have been published involving over 3,000 children from multiple countries, including those in Europe, Egypt, Turkey, India, Argentina, and China.19
In summary, although CMA currently ranks as one of the most common food allergies worldwide, diagnosis is hampered by the overlap of symptoms with other common childhood illnesses. Non-IgE-mediated CMA is particularly challenging to diagnose. Both under- and overdiagnosis of CMA can have negative consequences, underscoring the need for improved diagnostic tools to increase HCP awareness. Meyer stressed that an awareness tool does not remove the need for full clinical assessment and judgement but can help solve part of the diagnostic puzzle. CoMiSS is one such tool developed by experts in the CMA field and validated in numerous global studies.
CoMiSSTM to Increase Cow’s Milk Allergy Awareness: Lessons Learned
Katerina Bajerova
CoMiSS was developed as a clinical tool aimed at increasing the awareness of HCPs for the presence and intensity of clinical manifestations possibly related to cow’s milk (CM) intake.20 Bajerova reviewed the current supportive evidence behind CoMiSS and summarised vital learnings from the multiple studies conducted for further developing CoMiSS and recent experiences using the tool. This evidence base includes the 25 original published studies, plus one pooled analysis of three studies and two reviews.19
The evidence for CoMiSS in presumed healthy infants encompasses five studies.21-25 Across these studies, median CoMiSS in apparently healthy infants ranged from 3–4, while the mean CoMiSS was between 3.6 and 4.7.21-25 These average CoMiSS scores are markedly lower than the median score of 6–13 identified in 16 studies of infants exhibiting symptoms possibly related to CM.19 The high intra- and inter-rater reliability of CoMiSS confirms that no special training is required to use this tool reliably, noted Bajerova, which has important implications for its use in telemedicine consultations.24 In some infants not considered symptomatic by their caregivers, high CoMiSS may also help to flag the presence of underlying CMA. This was shown in a Spanish cohort presenting with a CoMiSS of ≥10 and ≥12, of whom 76% and 87.5% of infants had a positive result after undergoing open CM challenge.24
In total, 22 studies have presented data using CoMiSS in infants who are allergic and infants with symptoms suspected to be CM related.19 Studies evaluating the decrease of CoMiSS during dietary elimination of CM have shown this to be predictive of a reaction to an oral food challenge (OFC) to diagnose CMA.18 Specifically, a reduction of ≥50% of baseline CoMiSS was associated with positive OFC. Similarly, a low CoMiSS score of <6 was considered predictive of the absence of CMA.21,22 Bajerova went on to summarise clinical outcomes from studies examining the sensitivity and specificity of different cut-off values of CoMiSS to CMA.23,26-31 CoMiSS ≥12 predicted a favourable response to a CM-free diet; however, sensitivity ranged from 20–77% and specificity from 66–92%. She concluded that this range of sensitivity, specificity, and cut-off values suggests that CoMiSS may operate differently according to the study design and the type of presented symptoms.
Overall, the collective clinical evidence supports several key benefits of CoMiSS. A high baseline CoMiSS associated with a significant reduction during a CM elimination diet is specific and supports the diagnosis of CMA. CoMiSS also facilitates detailed symptom tracking before and during an elimination diet, as required for the management of CMA. From a practical perspective, it represents an easy-to-use and functional awareness tool. However, it is essential to note that CoMiSS cannot be considered a standalone diagnostic tool for CMA, cautioned Bajerova. Even if the baseline value is in the high-risk interval and a decrease in CoMiSS occurs after elimination, an oral food challenge test is still required to confirm a diagnosis of CMA.
Bajerova also acknowledged that, by definition, an awareness tool requires sufficient sensitivity to detect infants at risk, so some important points related to CoMiSS remain open for discussion. Considering the evidence in hand, reducing the current cut-off value of ≥12 or adding new items to increase sensitivity seem favourable. However, Bajerova pointed out that there are already 25 studies showing how CoMiSS operates in real life; changing the current parameters significantly, risks negating these results.
As the final take-home message, Bajerova proposed that, if there is a pathway to determining which infants are allergic to cow’s milk and which are not, then CoMiSS can be thought of as the compass that helps to navigate that pathway and reach the correct target: diagnosis of CMA.
Can CoMiSSTM Be Improved?
Yvan Vandenplas
CoMiSS was first developed in 2015 by an expert consensus panel and, since its conception, has been used globally in multiple clinical studies.20 In 2022, the expert consensus panel, including 7 original members, met in Lausanne, Switzerland, to debate the 2022 updates to CoMiSS, using the collective evidence to determine if the tool could be improved. The consensus paper resulting from this expert panel meeting has now been published. 32
The expert panel discussed and voted on several CMA manifestations, and Vandenplas shared the key points. The group agreed that many signs and symptoms of CMA can be seen in both IgE and non-IgE-mediated diseases and that this symptom overlap can make it challenging to recognize whether the allergy is IgE mediated or not. There was consensus that anaphylaxis, failure to thrive, and haematochezia should not be included as part of CoMiSS.
The age limit for CoMiSS was the focus of extensive panel debate. Arguments in favour of ≤6 months centred on the fact that data in the healthy population are limited to this age group. However, the counterpoint was also made that many infants are long-term breastfed so will not have any contact with CM proteins until after the age of 6 months. Overall, the group agreed that CoMiSS should preferably be used in infants ≤6 months but that this may be extendable up to a maximum of 12 months. There was complete agreement among the panel that CoMiSS should not be used beyond the age of 12 months. Another essential update to the CoMiSS score was that a revised cut-off of ≥10 was suggested as the new threshold value for the risk of CM-related symptoms; based on the 90th-percentile data published for the normal population.
Vandenplas noted that the expert group had engaged in extensive debate around acute urticaria, with the decision that “existing since at least 1 week” should be added to all symptoms, except for urticaria and angioedema. This clause was added to exclude all those acute situations in which the same symptoms may manifest. Urticaria was maintained in the updated CoMiSS, but angioedema was added to urticaria, and the exact weighting was kept for both (urticaria and/or angioedema [no: 0 or yes: 6]). Most of the expert group agreed that if urticaria/angioedema can be directly related to CM (e.g., drinking milk without any other food), this is strongly suggestive of CMA and may not need further CM challenge.
The existing scoring for crying/irritability, regurgitation, respiratory symptoms, and atopic eczema were left unchanged in the updated CoMiSS. One of the main reasons being that changes to any of these parameters would render the clinical evidence accumulated using the previous CoMiSS score invalid.
Regarding stool composition, the expert panel agreed that the Brussels Infant and Toddlers Stool Scale (BITSS) and the Bristol Stool Scale (BSS) could be used interchangeably in CoMiSS, according to HCP preference. While BSS was developed to evaluate GI transit in adults, BITSS was developed specifically for non-toilet trained children.33 Changing BSS to BITSS was found to have no clinical impact on the resulting CoMiSS score.34
In summary, CoMiSS constitutes a valuable awareness tool for evaluating CM-related symptoms in otherwise healthy infants aged 6 months or less, with this age limit extendable up to a maximum of 12 months. A revised CoMiSS cut-off score of ≥10 may be suggestive of CMA. Vandenplas clarified that this decision to decrease the previous CoMiSS cut-off of ≥12, proposed arbitrarily, to ≥10 was based on the accumulated body of clinical evidence.
Overall, CoMiSS remains an excellent tool to increase the awareness of HCPs to the possibility that the symptoms presented by infants are related to CM. Vandenplas concluded his presentation by showing the updated CoMiSS for 2022 (Figure 2) and calling on delegates to use this form to increase CMA awareness and avoid over- and underdiagnosis.

Figure 2: 2022 updated CoMiSSTM.32
Trademark of Société des Produits Nestle S.A., Vevey Switzerland © 2022 Nestlé.
All rights reserved.
Question and Answer Session
Rosan Meyer, Katerina Bajerova, and Yvan Vandenplas
The symposium presentations were followed by a question-and-answer session in which the expert panel responded to questions posed by both in-person audience members and delegates attending virtually.
A question from the online audience asked whether CoMiSS was suitable for use by health visitors and community nurses, or if modifications would be required for these grassroots HCPs. Bajerova replied that the tool can be used interchangeably and without problems. Vandenplas added that the practical applicability of CoMiSS had been clearly illustrated in a Spanish study, where the tool was used by parents and then independently filled in by HCPs, and there was no difference between the scoring.
A question from the live audience queried whether, during a literature review, a distinction was made between IgE and non-IgE-mediated CMA and the corresponding CoMiSS test performance. Bajerova replied that no difference was seen between IgE and non-IgE-mediated allergy when reviewing the clinical papers. Some studies included both groups of patients and CoMiSS performed equally well irrespective of the presence or absence of IgE.
A follow-up question from the same delegate flagged problems when using CoMiSS in daily practice regarding urticaria in non-IgE-mediated CMA since this is often not linked to CMA but somewhat related to viral infection or histamine hypersensitivity. Vandenplas acknowledged that the historical development of CoMiSS as a combination of IgE and non-IgE-mediated allergy had caused some confusion. To remedy this, the updated 2022 CoMiSS specifies that there must be a direct time relation between the ingestion of CM as a single food and the appearance of urticaria. This improvement clarifies the relationship and avoids confusion with other causes of urticaria, noted Vandenplas.
In response to a question on elimination diets and the impact of small protein oligopeptides in infants receiving hydrolysate or amino acid formula, Meyer acknowledged that approximately 10% of children on extensively hydrolysed formula, whether that is casein- or whey-based, may continue to react due to the residue of β-lactoglobulin. She added that CoMiSS is a valuable tool to assess the success of dietary elimination and, in published studies, “a very nice association” was seen between reduction of the score with elimination and reintroduction. In the initial stages of its development, CoMiSS was first used to assess the success of formula changes.
On whether parents can reliably use CoMiSS, the panel’s response was categorically “Yes.” However, Meyer cautioned that it was still crucial for HCPs to be involved in the CMA diagnostic process and apply their own clinical assessments.
Looking at the next steps for the CoMiSS expert group now that the score has been recently updated, Vandenplas explained that the first research priority is to gather more ‘normal’ data in the age groups between 6 and 12 months and to validate the score in healthy infants outside Europe. Up to now, the normal range has been based solely on evidence from European countries, and the CoMiSSTM cut-off should at least be confirmed that it remains the same outside Europe, he noted. Vandenplas invited audience members to join efforts to strengthen the CoMiSS evidence base by providing data from outside Europe, with studies that increase geographic representation or by carrying out research in infants from 6–12 months.
A member of the live audience commented that, anecdotally, clinicians working in GI and paediatric practice are seeing an increase in CMA diagnoses made by their GP colleagues. The delegate asked if there is population-based data on whether the incidence of CMA is increasing, or is it just being recognised and diagnosed more? Meyer replied that, although up-to-date prevalence figures from the UK are not available, an increase in prescribing of hypoallergenic formula has been observed, which may act as a proxy for CMA diagnoses. The last prevalence study was EuroPrevall in 2015 and, while the UK had the highest CMA rate at 1.3%, current national prescription databases indicate the true prevalence is much higher. “I think we have a problem with over prescribing,” said Meyer, “and the true prevalence of CMA in the UK is probably between 1.3% and 2%.”
A follow-up question asked whether, if CoMiSS is placed in the hands of GPs, it might further increase prescribing of specialist formula. In response, Meyer explained that GPs are “grateful” to have CoMiSS as it functions as a simple and quick awareness tool. She noted that one of the main problems in general practice is time constraints, which can make it difficult to bring patients back and rechallenge them. Meyer stressed that a tool like CoMiSS is helpful to navigate the diagnostic pathway to CMA, but part of that must still include the elimination diet and subsequent gold standard OFC.
Conclusion
Hania Szajewska
Symposium co-chair Szajewska concluded the meeting by summarising the key take-home messages.
The CoMiSS awareness tool has been well studied and may improve the CMA journey from symptom presentation to diagnosis for both patients and HCPs.
CoMiSS is an easy-to-use and practical awareness tool for evaluating CM-related symptoms but cannot be considered a standalone diagnosis tool for CMA.
Updates to the CoMiSS tool for 2022 now allow BITSS and BSS to be used interchangeably, and the cut-off score has been lowered to ≥10 to further improve sensitivity for symptoms suggestive of CMA.






