Abstract
A rare life-threatening condition has been noticed in children who have been previously infected with COVID-19, involving organ inflammation, named as a multisystem inflammatory syndrome. In this study, the authors analyse a unique case, describe possibilities of disease manifestation in a particular individual, and detail different treatment options. Along with treatment and monitoring, all eligible children should be vaccinated against COVID-19. Unfortunately, due to decreased availability and increasing demand for the COVID-19 vaccine, the government in Pakistan has been led to vaccinate the general population on the basis of age group. Currently, individuals older than 12-years-old are being vaccinated, but not those who are younger. As a result, younger children have a higher chance of being infected with COVID-19 and developing multisystem inflammatory syndrome in children (MIS-C). Vaccination against the virus reduces the likelihood of COVID-19 infection. MIS-C is a rare condition found in children that might be fatal, and current evidence indicates that MIS-C occurs due to exaggerated immune response.
Key Points
1. Every paediatrician should have basic knowledge about severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its related complications, as well as all aspects of the disease. Paediatricians should listen to their patient’s concerns, and provide best possible care in accordance with latest medical research and best practices.
2. This article presents a unique case of multisystemic inflammatory syndrome in children (MIS-C) secondary to SARS-CoV-2 and details the clinical manifestation of the illness, investigations, and management. It emphasises that healthcare professionals should investigate for the presence of multiple organ system manifestation. MIS-C is a potential diagnosis for every patient presenting with fever, rash, and systemic manifestations, along with Kawasaki disease and other illnesses.
3. MIS-C is a rare but life-threatening complication of SARS-CoV-2, with children being the most impacted by the disease. As a result of young children not being vaccinated against COVID-19,
MIS-C has become a leading concern. This study aims to provide valuable information about the disease and its prevention from complications.
INTRODUCTION
A novel characterised hyper inflammatory condition has been observed in children who have recently had severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections during the COVID-19 pandemic. Children with this condition had fever and general sickness, as well as occasionally shock, cardiac involvement, and multiple organ failure. After being discovered in the UK, reports of this ailment later spread to other regions of Europe and the USA.1-3 A study conducted in the UK showed a remarkable number of hospitalised patients who became extremely ill, and developed acute respiratory distress syndrome and multiple organ failure as a result of COVID-19. Multiple organ dysfunction might involve multiple organ systems.
A 61% increase in mortality rate was seen post-kidney failure in approximately half of the patients who developed acute respiratory distress syndrome after contracting COVID-19.4 Serious illness has been reported in children with the new syndrome, which occurred after COVID-19 infection, with clinical presentations resembling to that of Kawasaki disease and toxic shock syndrome.5 SARS-CoV-2 causes deregulated immune response that snuffs out antibodies and causes a drastic decrease in functional capacity, leading to insistent extrapulmonary infection.6
A study conducted in North America revealed that 45 patients of the paediatric age group (median age: 9) were admitted to hospital as a result of MIS-C. Cardiac involvement was apparent in nearly 80% of children, which involved coronary artery dilatation and reduced cardiac output. Most patients had raised levels of inflammatory markers, as well as raised cardiac biomarkers that suggested cardiac injury. Patients were managed with immunoglobulins, corticosteroids, and immune modulators to reduce the inflammatory reaction. One-third of the patients were treated with non-invasive breathing assistance; however, none of the patients required support from a ventilator. In addition, more than half of the patients were treated for low blood pressure. Furthermore, it was observed in the following study that the inflammatory markers and cardiac biomarkers had returned to its normal range tertian. Cardiac abnormalities, as well as coronary artery dilation, was not seen in patients after 4 months.7
CASE REPORT
A 9-year-old, previously healthy, female child presented in the Holy Family hospital, Karachi, Pakistan, in the outpatient department in September 2020, presenting with fever, abdominal pain, and vomiting for 2 weeks. The fever was sudden in onset, intermittent, and documented up to 103 °F; it was not associated with rigors and chill. Abdominal pain was generalised, episodic, and moderate in intensity; there were no aggravating or relieving factors. Vomiting accompanied the pain and was non-projectile. The patient had two episodes a day that contained food particles and it was not associated with bleeding. There was no history of earlier hospital admissions. The child lived in a home with four other family members, and there was no history of travel or contact with sick individuals. There were no known allergies. The patient took syrup acetaminophen to treat their symptoms.
On admission, the patient’s vitals were temperature: 101 °F; blood pressure: 97/63 mmHg; pulse: 100 bpm; respiratory rate: 20 bpm; saturation of peripheral O2: 96% at room air; and weight: 29 kg. The patient was alert and oriented with time place and person. Their Glasgow Coma Scale (GCS) was 15/15. Physical examination revealed generalised maculopapular rash all over their body, oral ulcers, and conjunctivitis. Pallor, cyanosis, clubbing, oedema, icterus, and lymphadenopathy were absent. The rest of the systemic examination was unremarkable; however, on cardiovascular examination, gallop sounds were observed while the respiratory system had no significant finding. There was no visceromegaly or tenderness present upon abdominal examination.
The patient was febrile on arrival, and intravenous paracetamol 1 g was given for fever. A broad-spectrum antibiotic, ceftriaxone, was given, with the dose of 1.5 g once daily. Intravenous fluid of half-strength dextrose saline was given as maintenance fluid.
Laboratory investigations were done, including baseline investigations, dengue antibodies, malarial parasite, urine detailed report, and blood cultures. The complete blood count revealed haemoglobin of 10.8 g per decilitre (10.9–14.9 g/dL), total leukocytes count of 18.1×109/L (4.5–14.5×109/L), neutrophils of 93% (38–68%), and lymphocytes of 5% (25–54% [Table 1]).8
Dengue antibodies and malaria parasite were negative. The urine report was insignificant. The initial blood culture and sensitivity was negative at 48 hours. The child had a recurrent fever spike of up to 102 °F. On the third day of admission, reverse transcription PCR and antibodies were sent to rule out the cause of underlying fever. The reports were PCR negative, and SARS-CoV-2 antibodies IgM and IgG were reactive.
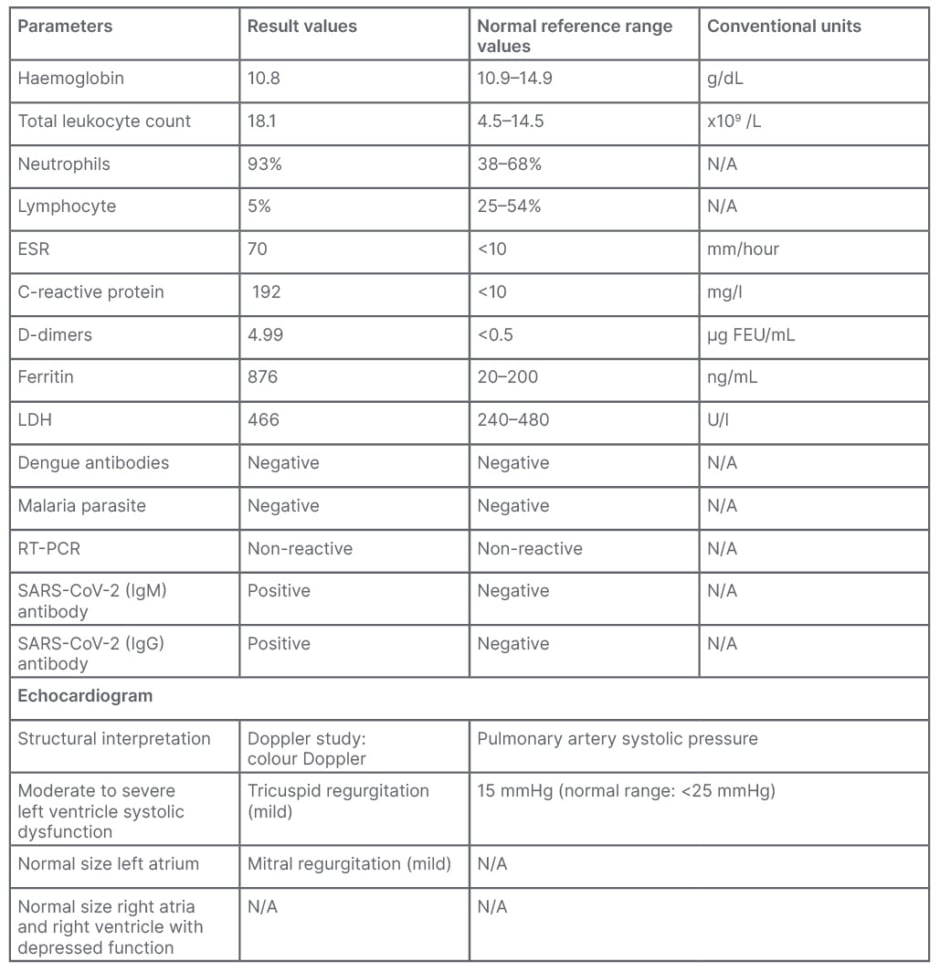
Table 1: Clinical data.8
ESR: erythrocyte sedimentation rate; FEU: fibrinogen equivalent unit; LDH: lactate dehydrogenase; N/A: not applicable; RT-PCR: reverse transcription-PCR; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Conclusion
Biventricular dysfunction; moderate to severe left ventricular systolic dysfunction; and ejection fraction of 30–35% inflammatory markers, including C reactive protein, erythrocyte sedimentary rate, D-dimer, lactate dehydrogenase, and ferritin were sent, revealing C-reactive protein of 192 mg/L (normal value: <10 mg/L), erythrocyte sedimentation rate of 70 mm/hour (normal value: ≤10 mm/hour) and D-dimer of 4.99 µg fibrinogen equivalent units/mL (normal value: <0.5 µg fibrinogen equivalent units/mL), while ferritin was 876 ng/mL (normal range: 20–200 ng/mL) and lactate dehydrogenase reported as 466 U/L (normal range: 240–480 U/L). Chest X-ray reports were unremarkable. Echocardiography revealed biventricular dysfunction, moderate to severe generalised left ventricular dysfunction, and ejection fraction of 35 percent. The patient was diagnosed with multisystemic inflammatory syndrome.
The patient was started on an injection of methylprednisolone (dose: 500 mg once daily for 5 days). Tablet aspirin (60 mg) was given once daily for venous thromboembolism prophylaxis, while tablet captopril (8 mg) and tablet furosemide (5 mg) were prescribed for three times a day for left ventricular dysfunction and reduced ejection fraction.
The patient was afebrile since the fifth day after admission, and was vitally stable. The patient was discharged on the sixth day after admission. They were discharged on oral antibiotic syrup cefixime (5 mL/200 mg) once daily for prophylaxis of bacterial infection, as the final blood culture report was still pending, and had to be reported 7 days after collecting the blood culture. Tablet prednisolone was prescribed (one tablet, three times a day). The authors continued to prescribe aspirin and captopril on discharge at the same dosage. The patient was advised for follow-up in 3 days, with the final blood culture report. The final blood culture came back as negative, after which the patient was no longer prescribed with antibiotics. See Table 2 and Figure 1 for the patient’s clinical course.
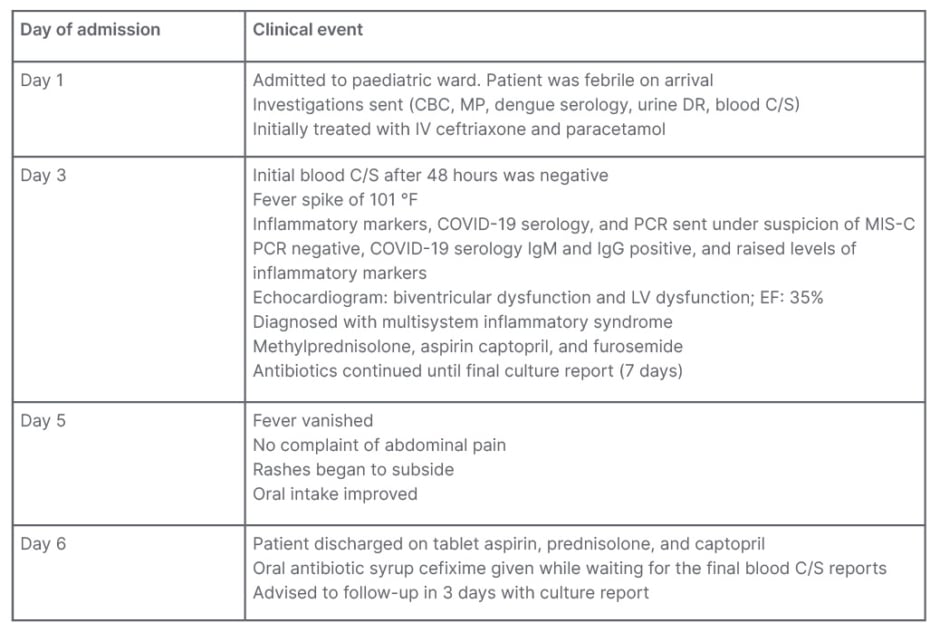
Table 2: Clinical course.
CBC: complete blood count; C/S: culture and sensitivity; DR: detailed report; EF: ejection fraction; IV: intravenous; LV: left ventricular; MIS-C: multisystem inflammatory syndrome in children; MP: malaria parasite.

Figure 1: Clinical course of a patient with multisystemic inflammatory syndrome in children.
CBC: complete blood count; C/S: culture and sensitivity; IV: intravenous; MIS-C: multisystem inflammatory syndrome in children; MP: malaria parasite; Temp.: temperature; UDR: urine detailed report.
DISCUSSION
MIS-C is a rare, fatal syndrome that affects individuals who have been infected with COVID-19. The authors’ case report highlights a patient who had no history of illness with prior SARS-CoV-2 infection, and then developing multisystemic inflammatory syndrome. Currently, evidence indicates that MIS-C occurs due to hyperbolic immune response, initiated by prior COVID-19 infection.9 In SARS-CoV-2, cellular entry occurs when viral proteins interact with multiple angiotensin-converting enzyme receptors represented by multiple organ system.10
Furthermore, the Centers for Disease Control and Prevention (CDC) defined MIS-C in five points. The first point was if an individual less than 21-years-old and presents with fever; second point mentions importance of diagnostic markers in making diagnosis, including C-reactive protein, fibrinogen, and erythrocyte sedimentation rate, which are markers of inflammation; the third point highlights clinical symptoms exhibition, critical ailment needing hospitalisation, and more than two organ systems entailment (cardiovascular, renal, respiratory, haematological, gastrointestinal, mucocutaneous, or neurological); the fourth point states that there should be no other alternative diagnosis; and the fifth point highlights that requirement of confirmed reverse transcription-PCR serology, antigen testing, or negative SARS CoV-2 test but exposure to a suspected or confirmed COVID-19 case within 4 weeks prior to onset of symptoms.1
The authors’ patient met the requirement bestowed in the case definition. There was history of fever, elevation of inflammatory markers, cardiac, gastrointestinal and mucocutaneous involvement, and positive SARS-CoV-2 serology. A study reveals that gastrointestinal involvement is the most common clinical manifestation in MIS-C, followed by cardiac manifestation, in the paediatric population.11
Maculopapular rash with fever can be brought on by a broad variety of illnesses, such as roseola, which causes high-grade fever, cough, coryza, and conjunctivitis, along with Koplik’s spot on buccal mucosa. Moreover, rubella, which shows painful adenopathy in the posterior auricular and posterior cervical lymph nodes, and relates to petechiae on the soft palate, is another infectious condition that may present with rash and fever. Additionally, Kawasaki disease also presents with diffuse rash, which spares the face but does have conjunctivitis of both eyes; cervical lymph nodes involvement may also be seen. Lastly, a patient with Kawasaki disease could have strawberry tongue on oral examination. Furthermore, scarlet fever manifests as a rash that spares the face, palms, and soles of the feet. This condition also has a quick onset of a fever, lethargy, pharyngitis, and strawberry tongue. Lastly, some medications might cause maculopapular rash, which commonly appears 4–21 days after starting a new medication; the rash may also rapidly spread and be diffuse in character.
In the authors’ patient, there was a diffuse rash that covered the entire body, which was accompanied by a high-grade fever, but there was no pharyngitis, tonsillitis, or adenopathy. There were no findings on oral examination other than oral ulcers, and there was no history of starting any new medications, as per the CDC guidelines, as a result of multiple organ involvement, along with maculopapular rash, it was possible that the rash was caused by MIS-C.12
In the middle of 2020, the first case of MIS-C was reported in UK. The patient’s symptoms were a similar presentation of atypical Kawasaki and toxic shock syndrome, which was a manifestation of severe COVID-19.13 Kawasaki disease presents with acute inflammation of medium sized vessels in paediatric population under 5-years-old, and typically occurs between the ages of 9–11 months.14
Though the cause of Kawasaki disease remains unknown, the clinical presentation of COVID-19 illness resembles that of Kawasaki disease, occurring after an infection that leads to a cascade of inflammatory events,15 and leads to symptoms involving the gastrointestinal system. For instance, abdominal pain, reduced oral intake, and vomiting are more common in MIS-C compared with Kawasaki disease.16 Kawasaki disease and MIS-C have some factors in common, including prolonged history of fever, rash, oral mucositis, conjunctivitis, and raised inflammatory markers, as seen in the authors’ patient’s case. However, in 75% of the cases, Kawasaki disease involves a younger paediatric population, typically less than 5 years old; abdominal symptoms are less frequent; and multiple organ dysfunction is rare. Ventricular dysfunction is reported in acute cases of Kawasaki disease. In the authors’ case, the patient presented with left ventricular dysfunction, which is not typically seen in acute phases of Kawasaki disease. The child also had gastrointestinal symptoms, such as abdominal pain, whereas gastrointestinal symptoms are only present in 20% of the cases in Kawasaki disease.17
A previous cohort study conducted on children who tested positive with SARS-CoV-2 showed cardiac involvement in 13 out of 2,135 patients.18 A 2020 study also showed that patients with COVID-19 had a 16% advanced risk myocarditis compared with individuals who were not infected. Children under the age of 16 who were infected are at a 37-times higher risk of developing myocarditis, while the 16–39 age group are at a 7-times higher risk compared with individuals who are not infected with COVID-18.19
A large cohort study showed that individuals with MIS-C usually presented with tachycardia, and occasionally with hypotension, which led to increased mortality rate. This study also highlighted that 7% of cases resulted in decreased cardiac output because of left ventricular systolic and diastolic dysfunction. Furthermore, left ventricular diastolic dysfunction was also found in more than half of individuals.20 Another prospective case study conducted in France observed ejection fraction of as low as 10%.21 This suggests that myocardial dysfunction related to SARS-CoV-2 does not just affect children, but also adolescents and adults, making it a wide range viral infection. A study conducted in the UK and Italy highlighted important laboratory findings, suggestive of MIS-C with neutropenia, lymphopenia, thrombocytopenia, and elevated inflammatory markers, with raised levels of serum triglycerides, ferritin, and D-dimer. COVID-19 infection was not identified in a couple of patients following SARS-CoV-2 PCR, serology, and antigen testing.1,22
Managing MIS-C is similar to that of Kawasaki disease. The patients who present with mild symptoms in MIS-C are managed with an intravenous immunoglobulin infusion 2 g/kg, and the dose is repeated if there is resistance. The addition of other drugs, such as corticosteroids, cyclosporine, and biological agents (e.g., anti-IL-1) can reduce the risk cardiac manifestations such as coronary artery dilation.23 Patients with moderate to severe illness are treated with intravenous immunoglobulins, aspirin, and methylprednisolone. For those with moderate illness, the dose is 10–20 mg/kg/day for 1–3 days, followed by a maintenance dose; meanwhile, 30 mg/kg/day methylprednisolone is given to patients with severe disease for 3 days as a pulse therapy. Patients who have no response to two doses of intravenous immunoglobulins and steroids are managed with biological agents, such as anti-TNF and anti-IL-1. Although the role of anticoagulants in not well explained, enoxaparin can be given for thromboprophylaxis in MIS-C.16 Patients with MIS-C reported earlier were mostly managed in intensive care, and have been treated with intravenous immunoglobulins, steroids, TNF-α inhibitor.24
The patient in the authors’ case report recovered with supportive care and without being admitted to the intensive care unit. There was prompt improvement in patient’s condition after administrating steroids and anticoagulants, without being treated with immunoglobulins.
The incidence of COVID-19 is on the rise. Every child that presents with prolonged fever should be carefully assessed for multiple organ system manifestation, and should be carefully monitored in hospital. Empirical antibiotics were initially started, as clinical signs and symptoms of MIS-C resemble to that of other serious bacterial infections.25 Supportive care should be given. The CDC has recommended vaccination for children aged 6 months and older,26 as per the recommendations. Unfortunately, due to the decrease in availability and increasing demand of COVID-19 vaccine in Pakistan has led the government to vaccinate the general population on basis of age.27 On 28th September 2021, the National Command and Operation Center (NCOC) decided to start vaccinating children aged 12 and above, but not children below this age. This is the reason why younger children might be at a higher risk of becoming infected with COVID-19 and developing MIS-C, compared with adults.28
CONCLUSION
The incidence of COVID-19 on the rise, and every child who presents with prolonged fever should be carefully assessed for multiple organ system manifestation, and should be carefully monitored in hospital. Empirical antibiotics should be started, and supportive care should be given. MIS-C is a rare condition that can present with mild to severe illness. Most children improve clinically with medical care, while others rapidly deteriorate. Hence, the COVID-19 vaccine should be encouraged in the paediatric population falling in this age group, as recommended by CDC guidelines. The vaccine reduces the probability of contracting COVID-19; thereby reducing the risk of MIS-C.





