Abstract
The UK’s National Institute for Health and Care Excellence (NICE) has produced new evidence-based guidance on the prescription of cannabis-based medicinal products (CBMP) to treat epilepsy, chronic pain, spasticity, and intractable nausea and vomiting. For epilepsy, cannabidiol (CBD) (Epidyolex®, GW Pharmaceuticals, Cambridge, UK) is recommended in conjunction with clobazam for treating seizures associated with Lennox–Gastaut syndrome and Dravet syndrome in patients aged ≥2 years. Other CBMP covered by the NICE guidelines include the licensed product Δ-9-tetrahydrocannibinol (THC), combined with CBD (Sativex®, GW Pharmaceuticals, Cambridge, UK), nabilone, and synthetic compounds that are identical in structure to naturally occurring cannabinoids such as dronabinol. Understanding the clinical practice implications of these new NICE guidelines will enable clinicians to ensure patients that are suitable for treatment benefit from these important new treatment options, against a backdrop of increasingly avid patient and public interest. Optimal dosing, monitoring, and management of patient expectations will prove key to successfully implementing CBMP into the challenging clinical setting. It is also vital that doctors continue to draw a firm distinction between unregulated CBD products and licensed medications which have been rigorously tested in clinical trials. Unregulated products are associated with issues such as variability in active ingredient concentrations, labelling inconsistencies, impurities and contaminants compromising therapeutic efficacy, and risk of potentially exposing users to harm. This review summarises the new NICE guidelines on CBMP for severe treatment-resistant epilepsy, considers how guidelines will impact the management of epilepsy in clinical practice, and reiterates the key risks posed by unregulated cannabis-based products.
INTRODUCTION
The ‘treatment gap’ for people with epilepsy is a global health emergency; this is the number of people who need epilepsy treatment but are not in receipt of it, often because they live in a developing economy. However, treatment gaps are seen frequently, such as in the difference between those who take medication and those that receive the maximum benefit from it. The traditional model of epilepsy drug design, which includes both unanticipated and targeted discovery, has not made a meaningful impact on the proportion of people in Europe who are seizure free as almost 40% continue to have seizures.1 The epidemiological burden of treatment-resistant epilepsies should not be underestimated; collectively what is rare can be common. Indeed, Lennox–Gastaut syndrome (LGS), described as ‘wickedly common’, is thought to represent around 4% of paediatric epilepsies overall, but may account for as many as 1 in 10 cases presenting before the age of 5 years.2,3 LGS presents with multiple seizure types and various medications are required in an attempt to gain control of the disease.3 Dravet syndrome is also very common,2 and of every 500 children with epilepsy, it is estimated that two or three will have the syndrome.4 Therefore, a growing desire from patients and clinicians has arisen for a more innovative approach in the search for therapeutics. This drive has led research into implantable neurostimulators, refined surgical procedures, dietary manipulation, and novel sources for medications.
The surge of interest in cannabis-based medicinal products (CBMP) is unmatched by any other antiepileptic medication, and consequently, patient advocacy groups have been involved from the very start. The preclinical data needed before commencing randomised controlled trials was performed in parallel with many anecdotal reports from parents who had been administering a variety of cannabis products to their children, reporting some benefits. This level of community engagement brings with it much frustration for parents as all new medications come at a research and design cost, and Epidyolex® (GW Pharmaceuticals, Cambridge, UK) is no exception. Epidyolex (Epidiolex US) is a highly purified liquid containing cannabidiol (CBD) derived from the cannabis plant.5 Prudent prescribing will ensure that certain patients are excluded from receiving CBMP, leaving some parents and their clinicians feeling aggrieved. In the UK, the policy is changing rapidly, and prescribing guidelines are evolving to keep up with this shifting landscape. Discussed here are the guidelines and regulatory framework behind currently available CBMP.
NICE GUIDANCE ON CANNABIS-BASED MEDICAL PRODUCTS: FOCUS AND EPILEPSY
The National Institute for Health and Care Excellence (NICE) has recently turned 21 years old and continues to be a commendable yet often criticised fixture of the UK healthcare landscape. NICE comes under the auspices of the Department of Health and Social Care (DHSC) and covers the National Health Service (NHS) in England and Wales, but not Scotland. NICE publishes guidelines on health technology and clinical practice and its role in the NHS is characterised by the need for its recommendations to be economically viable, with a focus on the quality-adjusted life year (QALY). A QALY is calculated as the change in utility value induced by the treatment multiplied by the duration of the treatment effect.6 If an intervention provided perfect health for 1 additional year, it would produce one QALY.6 NICE would only recommend an intervention if the incremental cost-effectiveness ratio was <£20,000 (€22,700) per QALY. Nonetheless, NICE is respected internationally for its robust recommendations that often pass the test of time.6
Recognising the need to help physicians navigate the emerging field of CBMP, NICE has recently produced new guidelines covering the use of these medications in four key indications: intractable nausea and vomiting, chronic pain, spasticity, and severe treatment-resistant epilepsy.7 This guidance is aimed at healthcare professionals, as well as individuals taking CBMP, their families, and carers.7 At the start of 2020, Epidyolex was only available to a very limited number of patients in the UK on a named patient basis or as part of a clinical trial; however, Epidyolex is now licensed for use in two rare, early-onset forms of epilepsy (LGS and Dravet syndrome).5
In developing the guidance on CBMP use in epilepsy, NICE reviewed evidence from four randomised controlled trials and 11 observational studies.8-22 Their recommendation was that Epidyolex could only be prescribed in conjunction with the long-acting benzodiazepine clobazam, as it appears more efficacious when the two drugs are co-prescribed. In LGS and Dravet syndrome, double-blind, randomised controlled trials with Epidyolex were carried out on a background of standard antiepileptic medication, most commonly clobazam (in approximately 60% of patients with Dravet syndrome and 50% of those with LGS) and valproate. It produced a significantly greater median monthly decrease in seizure frequency versus placebo, with significantly more patients achieving a >50% reduction in convulsive or drop seizures.9-12
To clearly define the efficacy of Epidyolex, NICE have extrapolated, post hoc, from trials that were not designed to answer the clinical question: does Epidyolex have to be prescribed with clobazam? This leaves clinicians in a conundrum; there is a paucity of high-quality evidence and yet we must navigate a clinically acceptable, evidence-based course. Questions from clinicians include: how much clobazam to prescribe and how often, when to change a patient from clonazepam to clobazam, what to do for patients who rely on clobazam being used sparingly as a rescue medication, what to advise for patients who have tried clobazam previously but experienced behavioural side-effects or intolerable sedation, does previous response to clobazam predict their sensitivity to Epidyolex, and if the clobazam is intolerable does Epidyolex prescription need to cease, and if so, how quickly? These questions could be addressed by well-coordinated open-label registries. A recent systematic review and meta-analysis looked at the link between CBD efficacy and clobazam status and found a significant boost in seizure response versus placebo when CBD was added to patients’ concomitant clobazam regimen. Rates of ≥50% seizure reduction were at 53% in ‘clobazam-on’ patients with LGS or Dravet treated with CBD compared to 28% in the placebo group (p<0.001). Comparative responses in the ‘clobazam-off’ arm were 29% for CBD versus 16% for placebo (p=0.015).23
Prescribing clinicians also need to be aware of potential drug interactions mediated via the cytochrome p450 system, the most clinically relevant of which are clobazam and valproate.5,24-26 CBD potentiates clobazam by increasing levels of its active metabolite N-desmethylclobazam and concomitant use may increase the incidence of transaminase elevations and clobazam side-effects.5,26 This may require down-titration of the clobazam dose or a move to liquid clobazam.5,26 Raised aspartate aminotransferase and alanine aminotransferase levels also commonly occur in conjunction with valproate use and may necessitate dose adjustments, or in rare cases, drug cessation.5,24 Routine monitoring of liver function tests is recommended for all patients prescribed Epidyolex at 1-, 3-, and 6-month intervals and periodically thereafter.5 Dosing of Epidyolex is weight-based and because of the liquid formulation small changes in dosing can be made, effectively personalising the dose for the patient. A standard starting dose may be 5 mg/kg/day for 1 week which may then increase to a maintenance dose based on response and tolerability.5
Currently, NICE recommends Epidyolex as an option for treating seizures associated with LGS or Dravet syndrome in patients aged ≥2 years.8,27,28 The frequency of drop/convulsive seizures must be monitored biannually and if not reduced by at least 30% compared to the 6-month period prior to starting treatment, then CBD should be discontinued.8,27,28 This guidance is summarised in Figure 1.29
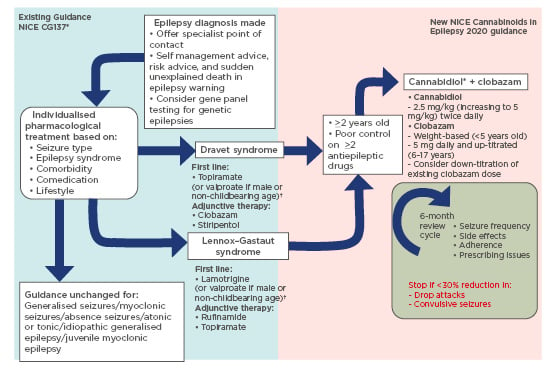
Figure 1: Changes to NICE epilepsy guidance for Lennox–Gastaut syndrome and Dravet syndrome.8,27-29
Steps shown on the blue background (left) represent existing guidance on current management of epilepsy. Steps on the pink background (right) represent new guidance on use of cannabis-based medicinal products in epilepsy treatment.
* Epidyolex remains the only recommended product by the National Institute for Health and Care Excellence (NICE) in the UK.
†Avoid valproate in women who may become pregnant during the treatment course.
NICE emphasise that these recommendations should not impact NHS-initiated treatment of CBD with clobazam that began before November 2019, which they advise can continue until it is deemed appropriate to stop.30 Although not yet supported by NICE, the British Paediatric Neurology Association (BPNA) also believes there is anecdotal, weaker evidence (from open-label, uncontrolled studies) suggesting a possible benefit of expanding Epidyolex prescribing to other intractable epilepsies in children and young people.24 Prescribing with a broader licence in other licensing territories should create a wealth of ‘real-world data’, enabling understanding of whether restrictive prescribing remains the best rationale. Therefore, carefully observing the experience of colleagues in North America will prove beneficial as they have had longer access to Epidyolex and conducted more open-label studies.31-34
WHY DOES NICE MATTER?
NICE plays an important role in standardising clinical practice and promoting value for money in the UK health system. This is relevant because there are remarkable health inequalities across the UK, as demonstrated by variances in epilepsy prevalence and premature mortality; there should not be additional postcode inequalities contributing to this in terms of available therapy.35,36 However, the division of England into autonomous clinical commissioning groups has created a secondary tier of rationing that may prevent clinicians from prescribing certain licensed drugs to patients.
The NICE clinical guidelines programme represents an important collaboration with the medical profession and thereby increases the likelihood of the ensuing recommendations being adopted;37 however, NICE guidance has some potential drawbacks.
One of the key principles of NICE is to ensure care is provided based on the best available evidence and, therefore, the quality and availability of existing evidence is pivotal. As the evidence evolves and new data accumulate, guidelines can rapidly become outdated and the process of updating them is both costly and time-consuming. Whilst guidelines are revised, plenty of innovation can occur in the interim. Furthermore, guidelines may act as a barrier to innovation, or discourage transformation of practices and demonstrations of excellence, and clinicians may also question whether guidelines really matter. Although the NICE epilepsy guidelines were not supportive of early use of levetiracetam, it has since gone on to become a blockbuster antiepileptic drug (AED) in the UK, accounting for >2 million prescriptions in 2019 (January–December).38 The structured process of evidence synthesis behind high-profile guidelines can identify significant evidence gaps and therefore be a springboard for innovation.
It is clear that evidence-based medicine can and does have a meaningful impact on prescribing behaviour. Analysis of 13.3 million patient-years of primary care records revealed changing trends, firstly in AED prescription over the last decade, with a shift from carbamazepine and phenytoin, towards lamotrigine and levetiracetam.
This change reflects published clinical data and an ever-expanding evidence base of AED efficacy, tolerability, and safety.39 It remains to be seen if this new NICE guidance on CBMP will exert a similar effect but it nevertheless fulfils the important role of putting the available evidence into context and addressing the increased interest from patients, the general population, and healthcare professionals alike (Figure 2).
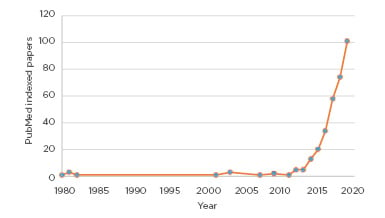
Figure 2: Interest in cannabidiol and epilepsy is booming.
Over 99% of published papers are younger than 10 years old.
This figure shows the count of unique PubMed entries for: “Epilepsy AND Cannabidiol” per year.
*Data for 2020 relates to the period of January–March, inclusive.
Guidelines can prove particularly valuable when a drug is new to a clinician. Despite the obvious ‘hype’ surrounding medicinal use of cannabis and cannabis extracts for people with epilepsy, individual clinician experience may still be limited. A recent cross-sectional survey of physicians treating children or adolescents with epilepsy across eight European countries showed that, although most doctors received requests for CBD on a regular basis, individual opinions about the optimal use of CBMP to treat epilepsy were diverse and disparate.40
EXPLORING THE IMPACT ON CLINICAL PRACTICE
NICE endorsement of CBMP for select patients with epilepsy is an important step forward and a helpful guideline for clinicians because of its scale of unregulated use. Already, the UK has an estimated 1.3 million regular CBD users, with 70% of these consumers pursuing formulations such as tinctures/oils or capsules in the quest for higher therapeutic doses of CBD.41 In the rationale for the guideline development, the NICE committee admitted that, despite the limited and low-quality evidence on the effectiveness of CBMP in epilepsy, they were spurred into action by “cases highlighted by stakeholders that individual patients have reported having fewer seizures with these medicines when other treatments have not fully controlled the seizures.”8
The new NICE guidance stipulates that the initial prescription of CBMP must be made by a specialist medical practitioner with a special interest in the condition being treated. The responsibility for prescribing and pharmacovigilance will then remain with this clinician for the duration of treatment. This makes transition agreements from paediatric to adult neurology even more crucial. The importance of the status of the prescriber is echoed by the Chief Medical Officer, in guidelines produced by the Royal College of General Practitioners (RCGP), the BPNA, and the Royal College of Physicians (RCP).24,42,43 The British Medical Association (BMA) has also produced a policy and research statement outlining the background to, and likely changes stemming from, increased prescribing of CBMP.44 Although issued as a prelude to the NICE guidance, the advice to prescribers from these collective professional bodies is in broad alignment, with emphasis on specialist prescription only for the small number of patients with an unmet medical need.
The licensing process in England differs from that of Scotland, but specialists everywhere are frustrated at being cast unwittingly as gatekeepers for this new drug. Even when the absolute block to prescribing may be at a licensing level, specialist clinicians may feel pressured to prescribe.
It is also important to acknowledge that many parents are desperate, having heard miraculous stories in the press, and deserve to be met with active, compassionate, and fully-informed engagement.25 Clinicians should always be mindful of instances where refusal to prescribe may lead the patient to seek a nonmedicinal product and therefore they should provide counselling and advice accordingly.
There are several logistical implications of CBD prescribing. Up until very recently in the UK, Epidyolex was a controlled drug and therefore prescriptions needed to be handwritten, using doses and quantities in numbers and figures. Handwriting prescriptions each month may create issues in organising delivery to or pick-up by patients: an issue of acute importance during the COVID-19 pandemic. Space in controlled drug cupboards may also be limited, so storage capacity will need to be expanded accordingly. Unlike other AED routinely prescribed for epilepsy management, the responsibility will be on patients to alert specialist prescribers in tertiary centres of their need to have the CBMP medication represcribed. This responsibility may increase the chance of accidental nonadherence, particularly as parents cannot be permitted to stockpile a controlled drug.
Management of patient expectations about CBMP is essential from the outset. There is a perception within the general population that plant-based products such as cannabis are ‘natural’ and less toxic than traditional drugs, offering a mild and benign safety profile.26,45 It is therefore important to advise patients of the risk of potential CBD-derived adverse effects, which may otherwise be erroneously attributed to other AED. Notable side-effects of Epidyolex include somnolence and sedation, which are increased in combination with clobazam and most common in the early stages of treatment.5 Gastrointestinal side-effects, specifically anorexia and diarrhoea, can also limit its use.5 NICE indicates that, before prescribing Epidyolex, it is essential that patients stop any nonprescribed cannabis, including over-the-counter, online, and illicit products.8
Physicians should also be alert to the fact that the placebo response with CBMP may be high, as well as the expectation and conviction that these drugs will work.26,45 In a placebo-controlled, double-blind study of CBD in Dravet syndrome, 24% of participants in the placebo arm reported a reduction of seizure frequency of >50%.10 Furthermore, the measurement of seizure frequency used in most trials relies on patient and family self-reporting, which remains inherently subjective and vulnerable to placebo effects.46 In the absence of a robust biomarker for seizure frequency or epilepsy control, clinical judgement and close monitoring is paramount. Recording details of treatment, clinical outcomes, and adverse effects using local or national registers (if available), and sharing this information across treatment centres, is also important to better steer clinical decision-making on CBMP moving forward.8
NICE report some important factors to consider when prescribing any CBMP. For all patients, key issues to be aware of include:8
- Current and past use of cannabis (including any over-the-counter and online products).
- History of substance misuse, including the illicit use of cannabis.
- Potential for dependence, diversion, and misuse (in particular with Δ-9-tetrahydrocannibinol [THC]).
- Mental health and medical history, particularly liver impairment, renal impairment, and cardiovascular disease.
- Potential for interaction with other medicines, for example central nervous system depressants and other centrally active drugs, AED, and hormonal contraceptives.
- Pregnancy and breastfeeding (although not applicable to people with LGS and Dravet syndrome).
For babies, children, and young people it is important to be mindful of any potential impact on psychological, emotional, and cognitive development, changes to structural and functional brain development, and potential issues with sedation.
Finally, in order to follow NICE guidelines and effectively prescribe Epidyolex for epilepsy, clinicians also need to improve the recognition and diagnosis of LGS and Dravet syndrome, the latter of which is relatively common and affects 1 in 20,000 births.26,47 This can be achieved by strengthening links with paediatric neurology departments, actively pursuing gene panel testing, and meticulously gathering the medical history.26 In contrast, LGS is much more of a challenge to diagnose, particularly in retrospect and in instances when there are either insufficient EEG recordings or the number retained for review are lacking. Nonetheless, it is important to identify LGS, because it accounts for a meaningful proportion of all childhood epilepsies.
REINFORCING THE RISKS OF UNREGULATED CANNABIDIOL PRODUCTS
Unlicensed cannabis-based products have experienced exponential growth in popularity over recent years and are now widely available from high-street and online retailers across the UK. The majority of products are marketed as health supplements, however, CBD oils are also seeing increasing popularity as a fitness product, claiming to aid post-exercise recovery and improve performance despite a paucity of evidence for either role.25,48,49 In a recent online poll, >75% of the UK public voiced support for the use of cannabis as a prescribed medicine.50,51 Whilst the new NICE guidelines represent a timely response to the current mood surrounding CBD products, it is important to draw a firm distinction between widely-available products and licensed medications such as Epidyolex. Patients should be advised that, although easily accessible, these CBD products lack quality assurance and should not be viewed as equivalent medicines.25 Table 1 outlines the licensed CBMP, and a selection of widely available cannabis-based products.8,10,52-59
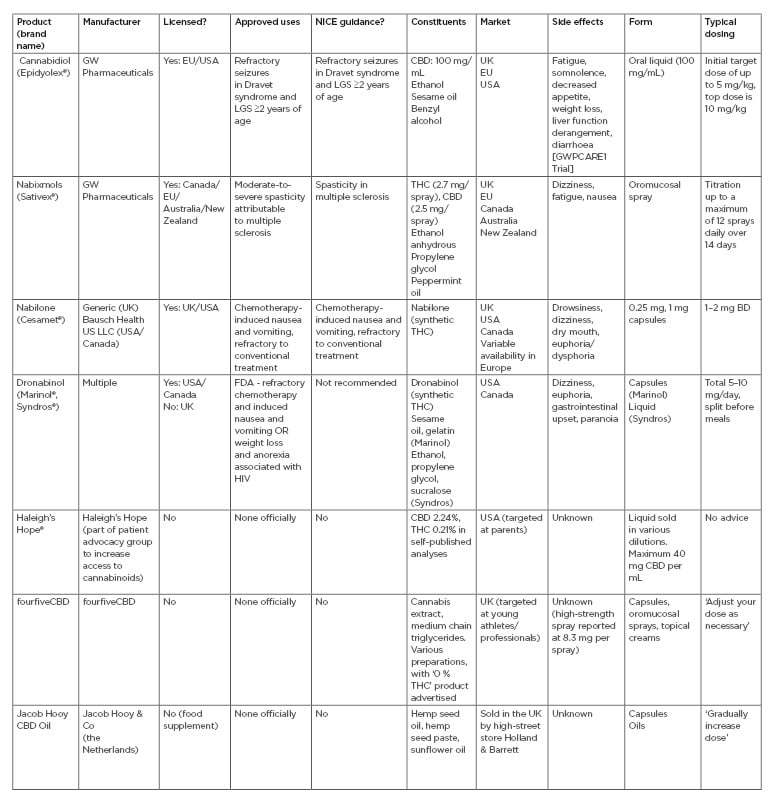
Table 1: Currently available cannabis-based products.8,10,52-59
Includes those licensed as medicines and a selection of more widely available unregulated products.
BD: twice daily; CBD: cannabidiol; FDA: U.S. Food and Drug Administration; LGS: Lennox–Gastaut syndrome; THC: Δ-9-tetrahydrocannibinol.
The Centre for Medicinal Cannabis (CMC), an industry body representing stakeholders in cannabis-based businesses, acknowledges that the current CBD sector in the UK is “profitable, competitive, and largely unregulated, with a diverse array of retail products and strong revenue growth.”41 The severely limited availability of legally-prescribed medicinal cannabis to date has helped to fuel this underregulated market for CBD products.41 People using CBD products for medicinal purposes are spending up to three times more a month than the general consumer population and, worryingly, the vast majority of purchases are made online (free from regulatory or clinical scrutiny).41
Although the safety profiles of licensed products like Epidyolex have been well-characterised in clinical trials, the same cannot be said for unregulated CBD products. It is important to ensure patients understand the key differences between CBMP and non medicinal products, the associated legal status, and the risk of harm or prosecution associated with them.25 NICE guidelines explicitly state the need for the efficacy and safety of CBMP to be monitored and evaluated, with adjustments to doses made by the initiating specialist prescriber as required.8 Clearly, this level of clinical vigilance cannot be applied in an unlicensed, uncontrolled setting, which creates the potential for poorly managed disease and/or unacceptable toxicity.
According to current NHS guidance on medical cannabis there is a “good chance” that the majority of products bought online will be illegal to possess or supply, as well as containing THC that renders them unsafe to use.60 While CBD boasts an excellent safety profile and is well tolerated, even at high doses, the THC component found in many unlicensed cannabis-based products carries an increased risk of serious adverse events.25,61,62 The principal risks of THC are dependency and psychosis, particularly in individuals who have schizophrenia or have a family history of psychotic illness.42,60,63 Other key THC-driven side-effects include disorientation and dizziness.61 There is also concern that chronic high exposure to THC can impact brain structure and development in both younger children and adolescents, potentially increasing risks of mental or physical health consequences.64
From an efficacy perspective, it is likely that all unlicensed CBD is subtherapeutic, creating an obvious barrier to effective seizure control.26 Any claims around health benefits are also a strict no-go as CBD is only considered medically beneficial when given in clinical doses and any measurable amount of THC in a product requires a licence from the UK Home Office.42 In direct contrast to claims advocated by some manufactures, there is currently no high-quality scientific or clinical evidence in humans to validate the suggestion that addition of THC to CBD increases the efficacy of CBMP as an antiepileptic therapy.24 A recent critical analysis of the available data concluded that lack of meaningful regulation of cannabinoid supplements continues to put consumers at undue risk without any clear evidence of therapeutic value.65 These concerns are mirrored in NHS guidance, which notes there is “no guarantee” that unlicensed cannabis-based products purchased from health stores will be of good quality or contain sufficient CBD to be therapeutically effective.60
Indeed, constituent variability and dose discrepancies are critical issues encountered in the unregulated CBD domain.42,66 Cannabis is an extremely complex plant and different strains can have completely unique CBD profiles.67,68 Many unlicensed cannabis preparations, such as artisanal cannabis oils, contain both THC and CBD in varying concentrations and proportions. These are not pharmaceutical-grade products and fail to comply with either good manufacturing practice or good distribution practice standards.24,26,66 Currently, there is no independent means for clinicians to quantify or characterise the content of such products, many of which contain significantly less CBD content and significantly more THC than labelled, compromising medicinal benefit and putting patients at risk of adverse events.24,26,66 The average amount of CBD found in unlicensed products is typically much lower (approximately 25 mg) than the 150–1,500 mg/day doses used in clinical trials.69-71 Even within the same product line, different batches may have different concentrations of ingredients and labelling may be incorrect.24 Dosage and bioavailability will also vary considerably depending on the formulation, strength, and purity of the product.42
These issues were highlighted in results from the first major blind testing study of 30 CBD oil products available online and from retailers in the UK.26,41 It revealed wide variability in quality, labelling inaccuracies, and the presence of controlled substances and contaminants (including dichloromethane and cyclohexane) and, in some cases, a complete absence of any CBD. Only around one-third of products were within 10% of their advertised CBD content and a further 38% contained less than one-half the labelled CBD concentration. Almost one-half (45%) of products tested had measurable levels of THC (>0.2 %), making them technically illegal within the UK.26,41 A further community-based study of patients self-supplying CBD revealed reported doses ranging from <0.5 mg/kg/day to 28.6 mg/kg/day, with THC doses between 0.0 mg/kg/day to 0.8 mg/kg/day.72 There have even been reports in the USA of poisoning after the consumption of a synthetic cannabinoid purporting to be CBD oil.73
CONCLUSION
Against the backdrop of escalating use of unlicensed cannabis-based products, NICE guidelines provide a solid, evidence-based benchmark for safe and effective prescribing of CBMP in epilepsy. By applying this guidance in the real-world clinical practice setting, with appropriate oversight and monitoring, neurologists can ensure patients with severe treatment-resistant epilepsy derive maximal benefit from CBD, while minimising the risks of harm.
However, when considering the clinical practice implications for epilepsy management, it is important to put the real-world scope and impact of these guidelines into context. Adults and children with LGS and Dravet syndrome collectively constitute <10% of patients with an active epilepsy, defined as experiencing seizures in any 12-month period and receiving antiepileptic medication. Consequently, the licensed use of CBMP is, for now at least, restricted in its relevance to only a small minority of the overall epilepsy population. Equally, the high-quality evidence that supports the efficacy of licensed CBMP is currently derived from just two specific epilepsy syndromes, so cannot be broadly extended to wider epilepsy management as a whole. Thus, while new NICE guidance undoubtedly marks an important step towards establishing a rightful place for CBMP within the overall therapeutic armoury, it cannot and should not be embraced as panacea for the treatment of all epilepsies.
With any new treatment advance or change in guidance, clinicians hold a privileged position as a key conduit for communication between the idealised world of randomised controlled trials and the reality of patients and their families living with epilepsy every day. As such, they have a vital role in communicating the guidelines that are received from professional bodies to patients and the wider community. Clinicians walk a tightrope between wanting to maximise potential benefit for patients, for example ensuring they have identified all eligible patients, without wanting to take on a promotional role for the pharmaceutical industry. Peer support and transparent, contemporary advice are great tools to enable them to achieve the key goal of individualising prescribing to the patient while also remaining responsive to guidelines and evidence.