Meeting Summary
This symposium took place on 10th October 2019, as part of the 28th European Academy of Dermatology and Venereology (EADV) Congress in Madrid, Spain. The symposium provided an overview of the advances in treatment of moderate-to-severe psoriasis over recent years, with a focus on IL-23 and IL-17 inhibitors. Prof Piaserico explained the mechanism of action of the new biologic therapies and their potential positive impact on both psoriasis and other comorbid conditions. Long-term disease control was identified as a continued unmet need which is of utmost importance to both the clinician and patient. Prof Piaserico described how psoriasis should be viewed as a marathon rather than a sprint. Prof Thaçi reiterated the need to review the efficacy and safety of biologic treatments following experience in everyday clinical practice, which can differ significantly from the data obtained from clinical studies. He emphasised the importance of obtaining a patient profile before initiating treatment and increasing adherence to treatment through regular patient reviews. The effects of the cessation of treatment on long-term disease was discussed. Prof Puig elicited from the symposium audience that their main concern regarding biologic use in moderate-to-severe psoriasis was the potential associated risk of infection. His presentation focussed on the safety data available for these therapies, both from national clinical registries and clinical studies, concluding that IL-23 inhibitors are associated with a significantly lower rate of overall adverse events than that of IL-17 inhibitors. The symposium concluded with a dynamic question and answer session.
Mind the Gap – The Benefits of Modern Biologic Treatment
Professor Stefano Piaserico
Advances in the treatment of moderate-to-severe psoriasis have accelerated considerably in the last 30 years. Prof Piaserico provided an overview of the pathogenesis of psoriasis. This begins with activation of dendritic cells by TNFα and IFNα, which is influenced by genotype, environmental factors, stress, and trauma. Activation of dendritic cells produces IL-23 which leads to differentiation of TH17 cells, Type 3 innate lymphoid cells (ILC3), and γδT cells. These cells produce IL-17, IL-22, and other proinflammatory markers which induce neutrophil and keratinocyte activation and proliferation. Keratinocytes produce other cytokines which recruit additional inflammatory cells (TH17, neutrophils, and macrophages), inducing a vicious circle which maintains the chronicity of psoriatic plaque formation.1-3
When psoriasis was discovered to be an immunologic disease, treatments involved broad spectrum immunosuppressive therapy, explained Prof Piaserico. TNFα inhibitors then became the mainstay of treatment. More recently, treatment has become highly specific, targeting IL-17, IL-12/23p40, and IL-23p19.1,4 Furthermore, studies have suggested the additional potential positive impact of IL-23 and IL-17 inhibitors on patients with depression, adipose tissue inflammation in obesity, and nonalcoholic fatty liver disease.5-9
Targeting IL-23 and IL-17: Impact on Patients
In the presence of IL-12, dendritic cells activate proliferation of Th1 cells, whilst activity of dendritic cells in the presence of IL-4 produces Th2 cells. The presence of IL-6 and TGF-β generates Th17-inducible cells, which can be modified in the absence of IL-23 into nonpathogenic Th17 cells which produce IL-17 and IL-10. In the presence of IL-23, Th17 inducible cells can be modified into pathogenic Th17 cells which produce IL-17 and IL-22.9-11
Prof Piaserico explained the mechanism of the IL-23/Th17 axis using the analogy of a cascade (Figure 1).12 Blocking the IL-17 receptor (e.g., with brodalumab), a downstream target, has a fast onset of action, but more frequent dosing is required, and quick relapse may occur when the drug is stopped. Blockade of IL-17 itself (e.g., with ixekizumab or secukinumab), a midstream target, results in a slightly slower onset of action than IL-17 receptor blockade; however, less frequent dosing is required. Blocking IL-23 (e.g., with tildrakizumab, guselkumab, or risankizumab), an upstream target, blocks the entire cascade with less frequent dosing required, which may be more convenient for the patient. The onset of action may be slower, but there may be the potential to modify the underlying disease process. In addition, IL-23 blockade may have a positive impact on inflammatory bowel disease because nonpathogenic Th17 cells are still present, allowing production of IL-17 which preserves the intestinal epithelial barrier despite its potential to drive pathogenic inflammation.13 The risk of candidiasis may also be lower with IL-23 inhibitors.14 There is a theoretical benefit of IL-23 inhibitors in spondyloarthritic disease, due to the effect on IL-22 which enhances new bone deposition in psoriatic arthritis.15
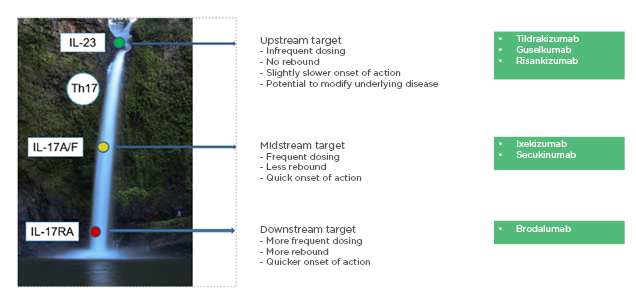
Figure 1: Cascade – the IL-23/TH17 axis in the pathogenesis of psoriasis.
Reproduced with permission from Prof Piaserico, EADV 2019.12
Unmet Needs
Despite advances, Prof Piaserico highlighted that there are still unmet needs: in particular, long-term disease control. One study has shown that patients rate maintenance of response to treatment over time as one of the most important drug characteristics, rating it higher than rapidity of response after initiating treatment.16 Pooled data from two randomised controlled Phase III trials studying the efficacy and safety of tildrakizumab (reSURFACE 1 and reSURFACE 2) showed that efficacy was maintained in Week 28 responders (≥75% improvement in Psoriasis Area and Severity Index [PASI 75]) through to 3 years.17,18
After long-term control, a decision must be made regarding a long-term treatment plan. In reSURFACE 1, PASI 75 responders who received their last dose of tildrakizumab at Week 16 showed a median time to relapse of 32 weeks (tildrakizumab 100 mg) or 36 weeks (tildrakizumab 200 mg).17,18 Prof Piaserico highlighted that this demonstrates control in >90% of patients 7 months after the last dose. A long-term adalimumab open-label extension study showed that when adalimumab was withdrawn in the subgroup of patients with stable psoriasis control (n=285), relapse occurred in approximately 60% of patients (n=178), with a median relapse time of 141 days.19
Data are lacking regarding the tapering down of treatment. Mostly uncontrolled emerging data indicate that a dose reduction of TNFα inhibitors can be achieved in a relevant proportion of patients with rheumatoid arthritis without losing clinical efficacy.20,21 Tapering down treatment reduces cost and dose-dependent side-effects.22-25
En Route to Long-Term Control?
Professor Diamant Thaçi
Prof Thaçi began his presentation by asking the audience what the most important factor is, for them as a physician, in the treatment of moderate-to-severe psoriasis. They voted in the majority for remission. Prof Thaçi acknowledged that review of available evidence often does not help distinguish which treatment has the greatest long-term efficacy.26 Head-to-head trials help guide clinical practice; however, results may differ in everyday practice as the population responds differently to subjects in the clinical trial, which have often been selected according to previous treatments.
Differing rates of drug survival are seen in clinical registries. Drug survival reflects a drug’s effectiveness, safety, and tolerability. The British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR) assessed the drug survival of biologics used to treat psoriasis in a prospective national pharmacovigilance cohort. Multivariate analysis showed that factors including female sex, being a current smoker, and a higher baseline dermatology life quality index were predictors of discontinuation. Presence of psoriatic arthritis was a predictor for drug survival.27 Drug survival has also been assessed from the Danish registry DERMBIO (Danish Biologics Interventions Registry), which collects data on all Danish patients with moderate-to-severe plaque psoriasis treated with biologics.28 Analysis of data collected between 1st January 2007 and 31st March 2017 showed that ustekinumab was associated with the highest drug survival and secukinumab with the lowest, although most patients on secukinumab were non-naïve. Switching from originator to biosimilar had no significant impact on drug survival, and the safety profiles were comparable. Adverse events occurred most frequently with secukinumab.28
Prof Thaçi acknowledged that his patients most appreciate continuous improvement without loss of response. Continuous improvement can be encouraged by seeing patients regularly to encourage treatment adherence.
Gone but Not Forgotten
Tildrakizumab was the first IL-23 inhibitor that Prof Thaçi had experience with. In two Phase III trials, reSURFACE 1 and reSURFACE 2, tildrakizumab 200 mg and 100 mg were efficacious compared with placebo and etanercept, and were well-tolerated in the treatment of patients with moderate-to-severe chronic plaque psoriasis.17
With TNFα inhibitors, we have learnt that disease improves but is still present: ‘gone but not forgotten’. reSURFACE 1 and reSURFACE 2 demonstrated a maintained improvement in PASI scores through to Week 148, without the fluctuations that are often experienced with other treatments.17 This effect seems to be common with all IL-23 inhibitors.
Stopping treatment with IL-23 inhibitors after long-term control seems to produce an effect which cannot be explained pharmacokinetically. The disease recurs but in a very slow manner. The median time to relapse after stopping treatment with tildrakizumab 100 mg is 224 days, or 252 days with tildrakizumab 200 mg (p=0.09).18
Similar results have been seen with guselkumab in two Phase III studies, VOYAGE 1 (N=837) and VOYAGE 2 (N=992). These randomised controlled trials demonstrated that guselkumab was superior to adalimumab in improving health-related quality of life, which was associated with greater skin clearance.29
Understanding psoriasis pathogenesis is still a puzzle but the pieces are slowly coming together, explained Prof Thaçi. The phenotype of the disease is changing but we are learning more about how to manage this systemic disorder. Psoriasis comorbidities are relevant for dermatologists when managing patients. It is important to consider the inflammatory processes affecting the skin and systemic symptoms, as well as the cardiovascular and psychiatric comorbidities. Quality of life and burden of disease must be reviewed when assessing treatment efficacy and patient satisfaction.30
Targeting Safety in Treatment Decisions
Professor Luis Puig
Prof Puig began by eliciting from the audience that their main concern regarding biologic use for moderate-to-severe psoriasis is the associated risk of infection. He reassured the audience that there is good safety data available to support the use of biologics.
There are many cytokines involved in the immune defence system against infections (Figure 2).14 IL-12 blockade may be harmful, with particular concern regarding infections such as tuberculosis and salmonella because IL-12 acts on both innate and adaptive lymphoid cells to produce IFNγ, which has a key role in preventing tumour initiation, growth, and metastases.14,31 IL-17 receptor blockade may lead to an increase in infections such as Staphylococcus aureus and Candida albicans.14 IL-23 blockade may be beneficial as it is known that IL-23 is overexpressed in human carcinoma and it can promote tumour growth indirectly.14
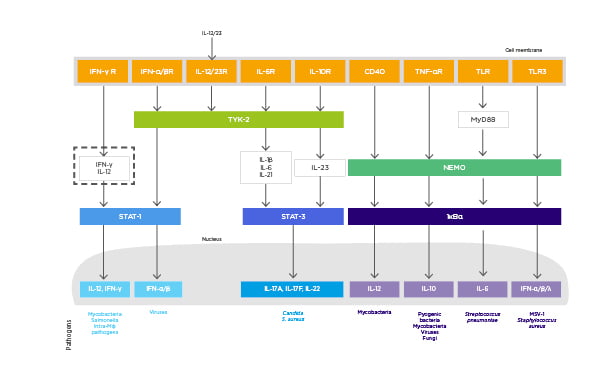
Figure 2: The role of cytokines in the pathogenesis of psoriasis and immune defence against infectious agents.
CD: cluster of differentiation; HSV: herpes simplex virus; Mφ: macrophage; NEMO: nuclear factor-kB essential modulator; STAT: signal transducer and activator of transcription; TYK: tyrosine kinase.
Adapted from Blauvelt et al.14
There are several comorbidities associated with psoriasis, for example, dermatological malignancies, myocardial infarction, and diabetes.32 Diabetes has the highest attributed risk in severe psoriasis, at 3.67 per 1,000 person-years. The attributed risk for melanoma is 0.05 per 1,000 person-years, with a number needed to harm of 20,135 patients due to the low prevalence of melanoma overall in the population.32 The main risk factor for myocardial infarction is age, so the number needed to harm is much higher in younger patients.32
Safety Data from Clinical Registries
Prof Puig summarised available registry data comparing safety of biologics for psoriasis. PSOLAR (Psoriasis Longitudinal Assessment and Registry), PsoBest (German Psoriasis Registry), and BIOBADADERM (Spanish Registry of Adverse Events Associated With Biologic Drugs in Dermatology) have collected data comparing safety of treatment with TNFα inhibitors and IL-12/23 inhibitors.33-35 Between 2008 and 2013, 1,030 patients received biologics and 926 patients received classic systemic treatment. The age- and gender-adjusted hazard ratio (HR) of adverse events was lower in the biologics group (HR: 0.60; 95% confidence interval [CI]: 0.5–0.7), with no difference in rates of serious and mortal adverse events. There was an increased raw relative risk (1.5; 95% CI: 1.2–1.8) of infections and infestations in patients who had received biologics.33 The overall risk of adverse events was not higher in the elderly (≥65 years, 9.8% of patients on the registry, drug group-adjusted HR: 1.09; 95% CI: 0.93–1.3), however serious adverse events were more common in the elderly (drug group-adjusted HR: 3.2; 95% CI: 2.0–5.1). Age-adjusted HR of all adverse events was lower for patients exposed to biologics compared to classic drugs (HR: 0.7; 95% CI: 0.6–0.7). Age did not seem to modify the effect of therapy (biologic versus classic) in the risk of adverse events.34 The BIOBADADERM registry has shown that combination treatment with methotrexate generally increases infection risk.35
PsoBest showed that in a total of 2,444 patients (1,791 patients on conventional systemic drugs, 908 patients on biologics), there was no significant difference in the risk of severe cardiovascular events between single conventional and biologic treatments.36
Data were analysed from 11,466 patients in PSOLAR at dermatology centres. A higher risk of serious infections was seen with adalimumab and infliximab compared with nonmethotrexate and nonbiologic therapies; however, no increased risk was seen with ustekinumab or etanercept.37 PSOLAR has also analysed data regarding risk of malignancy. Methotrexate and ustekinumab were not associated with an increased risk of malignancy, however treatment with TNFα inhibitors was associated with a significantly increased risk of malignancy over a 24-month exposure period.38
Clinical Study Safety Data
Clinical studies also provide important safety data regarding biologic treatment, as reviewed by Prof Puig. A systematic review and meta-analysis has shown that there is no significant difference between biologics and placebo in the risk of serious infection in patients with psoriasis at Weeks 12–16 (overall pooled Peto odds ratio: 0.71; 95% CI: 0.36–1.41) and Weeks 20–30 (odds ratio: 2.27; 95% CI: 0.45–11.49).39 Prospective cohort study data of low quality suggests that only adalimumab (adjusted HR: 2.52; 95% CI: 1.47–4.32) was associated with a significantly higher risk of serious infection compared with retinoid and/or phototherapy in adults.39
In a cohort of adult Kaiser Permanente Northern California health plan members with psoriasis diagnosed from 1998 to 2011 and treated with at least one systemic antipsoriatic agent, malignancy rates were calculated, adjusting for presence of psoriatic arthritis, prior ultraviolet light therapy, BMI, and cigarette use.40 Overall incident cancer rates were comparable between ever-biologic as compared to nonbiologic users. Nonmelanoma skin cancer rates were 42% higher among individuals ever exposed to a biologic, largely driven by increased cutaneous squamous cell carcinoma risk.
An observational cohort study was conducted using medical and outpatient pharmacy claims from two large USA health insurance claims databases from 2003 to 2015. The pooled propensity score-matched analysis yielded a decreased rate of overall serious infection in users of apremilast, etanercept, and ustekinumab, compared with methotrexate.41
A pooled safety analysis of IL-17 and IL-23 inhibitors has shown that IL-17 inhibitors are highly efficacious but are associated with a significantly higher rate of overall adverse events than that of IL-23 inhibitors.42,43 It may therefore be harder to achieve a good balance between safety and efficacy with IL-17 inhibitors. ECLIPSE, a Phase III randomised controlled trial, showed superior long-term efficacy with guselkumab, based on PASI 90 at Week 48, when compared with secukinumab for treating moderate-to-severe psoriasis.44 However, proportions of patients with adverse events, infections, and serious adverse events were similar between the two treatments.
Two Phase III randomised controlled trials, reSURFACE 1 and reSURFACE 2, assessed the long-term efficacy and safety of tildrakizumab in moderate-to-severe psoriasis for up to 148 weeks.17 Tildrakizumab was well-tolerated. Safety in the tildrakizumab 100 mg and 200 mg groups was compared with the etanercept 50 mg group. The main difference in the 148–week cumulative exposure–adjusted incidence rates of adverse events was in the rate of injection site reaction in terms of events per 100 patient years of exposure (1.94 with tildrakizumab 100 mg; 2.30 with tildrakizumab 200 mg; 40.41 with etanercept).17 Another two randomised controlled trials, UltlMMa-1 and UltlMMa-2, showed similar treatment-emergent adverse event profiles between ustekinumab and risankizumab, with no unexpected safety findings.45
Prof Puig ended by reiterating that class-specific adverse events occur less frequently with IL-17 and IL-12/23 inhibitors than with TNFα inhibitors, however paradoxical reactions have occurred with IL-17 and IL-12/23 inhibitors. Available data from IL-23p19 inhibitors have not shown an increased risk of specific infections or malignancies, however long-term data are needed to confirm their safety profile.46
Question and Answer Session
Q. Prof Thaçi queried which are the main factors to consider regarding safety when choosing a treatment.
A. Prof Puig reiterated the need to assess the patient profile when choosing a treatment. For example, in the case of anti-TNFα agents, patients should be screened for serious infection, for example hepatitis B and Mycobacterium tuberculosis, and active infections should be monitored closely.
Q. Are you expecting that the safety of IL-23 inhibitors will be different to that of anti-TNFα agents, asked Prof Thaçi?
A. Prof Puig explained that in his opinion, IL-23 inhibitors are preferred in a patient with a history of active neoplasms due to the risk associated with IL-12 inhibitors and skin cancer, and the potential link between TNF dysregulation and cancer.
Q. Prof Thaçi asked, in a patient with a chronic syphilis infection, which treatment would you use?
A. I would use an IL-23p19 inhibitor, responded Prof Puig.
Q. Is Candida infection a fear particularly associated with treatment, asked Prof Thaçi?
A. Candida infection is bothersome but can be treated with fluconazole. The patient can be profiled (usually diabetic, obese, previous history of relapsing Candida, or female) and treated prophylactically, responded Prof Puig.
Q. Do TNFα inhibitors have a place in the future for short- or long-term management of psoriasis, as many centres are forced to use TNFα inhibitors or use them in psoriatic arthritis?
A. TNFα inhibitors have a place in healthy biologic naïve patients. Special consideration is required for patients with heart failure and advice from a cardiologist may be required, responded Prof Piaserico and Prof Puig.
Q. How are dosages up or downtitrated?
A. You can downtitrate by increasing the dose interval or lowering the dose of the drug if drug dosages are not fixed, explained Prof Piaserico.
Q. Do you expect higher rates of malignancies if you increase the dose of IL-23 inhibitors from 100 mg to 200 mg?
A. No, there is no dose-effect shown in clinical trials, confirmed Prof Puig.
Q. Prof Piaserico, do you screen for nonalcoholic fatty liver disease in daily practice?
A. We only screen patients for the purpose of clinical studies, responded Prof Piaserico. There are some data showing that IL-17 inhibition plays a major role in reducing the progression of nonalcoholic fatty liver disease.
Q. How would you screen a patient before prescribing an IL-23p19 inhibitor?
A. Prof Puig responded that he screens all patients before making a treatment decision. However, if he had only IL-23p19 inhibitors available, he would not screen at all.
Q. Do you think IL-23 inhibitors should become the first choice of biologics in the future?
A. The speakers agreed that treatment choice must be guided by efficacy, safety, cost, and local regulations.
Chairman’s Closing Comments
Prof Thaçi presented the case of a 65-year-old woman who has been afflicted by psoriasis for several years. She does not have psoriatic arthritis but has diabetes and depression. Topical treatments and methotrexate have been ineffective. She has a previous history of malignancy (nonmelanoma skin cancer) and genital candidiasis. The audience were asked which treatment they would choose. The majority responded that they would use an IL-23 inhibitor. Prof Thaçi highlighted that this is a case-by-case decision and several treatment options are required to ensure tailored therapy, taking the patient profile and patient’s preference into account. In the case of psoriatic arthritis, it is important to consider consulting a rheumatologist. Prof Piaserico and Prof Puig advised that when switching biologics, they start the new biologic almost immediately. Prof Thaçi pointed out that it is important to consider side-effects or active infection when changing biologics.







